Chapter 4-Chemical Dominoes
advertisement
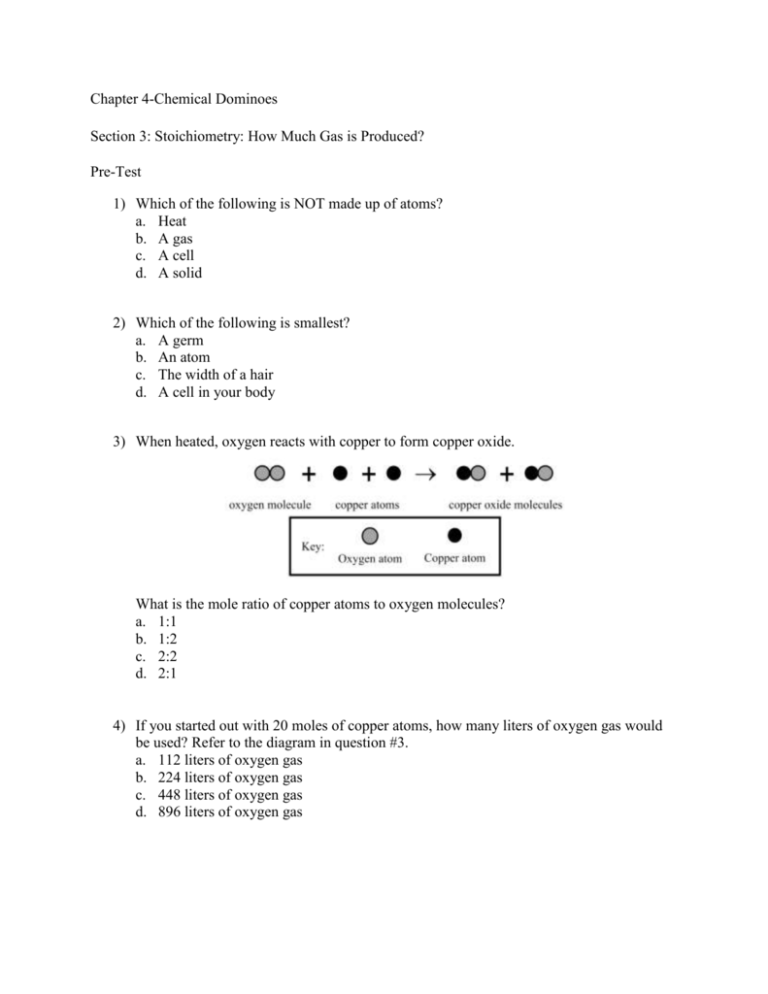
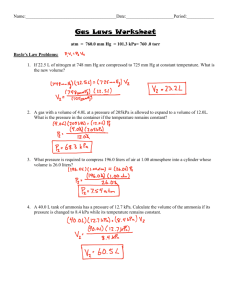
Chapter 4-Chemical Dominoes Section 3: Stoichiometry: How Much Gas is Produced? Pre-Test 1) Which of the following is NOT made up of atoms? a. Heat b. A gas c. A cell d. A solid 2) Which of the following is smallest? a. A germ b. An atom c. The width of a hair d. A cell in your body 3) When heated, oxygen reacts with copper to form copper oxide. What is the mole ratio of copper atoms to oxygen molecules? a. 1:1 b. 1:2 c. 2:2 d. 2:1 4) If you started out with 20 moles of copper atoms, how many liters of oxygen gas would be used? Refer to the diagram in question #3. a. 112 liters of oxygen gas b. 224 liters of oxygen gas c. 448 liters of oxygen gas d. 896 liters of oxygen gas 5) The diagrams below represent the combustion of propane with oxygen. This reaction produces carbon dioxide and water. How many liters of carbon dioxide gas would be produced from 10.0 moles of oxygen gas? a. b. c. d. 134 liters of carbon dioxide 168 liters of carbon dioxide 280 liters of carbon dioxide 373 liters of carbon dioxide Chapter 4-Chemical Dominoes Section 3: Stoichiometry: How Much Gas is Produced? Pre-Test Answer Key 1) 2) 3) 4) 5) A B D B A