Bond Energies Notes

Name: ____________________________________________ Period: __________ Date: __________________
Learning Target:
I can use thermochemical equations to calculate energy changes that occur in chemical reactions and classify reactions as exothermic or endothermic.
Criteria for Success:
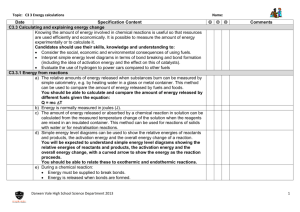
I can use average bond dissociation energies to calculate the enthalpy change for a chemical reaction.
Covalent Bond Energies and Enthalpy
A. The enthalpy change for a reaction, ΔH rxn
, can also be understood in terms of bonds ____________________ and
____________________ during a chemical reaction.
1. To break bonds, energy must be __________________to the system (endothermic).
2. To form bonds, energy is ______________________ (exothermic).
3. The following expression can be written to express this relationship:
ΔH rxn
= Ʃn(ΔH bonds broken
) - Ʃn(ΔH bonds formed
)
OR
ΔH rxn
= Ʃn(ΔH break reactant bonds
) - Ʃn(ΔH form product bonds
) a.
Notice that if the energy required to break the bonds is greater than the energy released in the formation of new bonds, the sign of ΔH rxn
will be _________________________ (endothermic). b.
Notice that if the energy required to break the bonds is less than the energy released in the formation of new bonds, the sign of ΔH rxn
will be _________________________ (exothermic). c.
It often helps to draw the ______________________ structures of the compounds involved in the reaction to determine the bonds being broken or formed. d.
You will be provided with the bond energies in the problem or be asked to look at a _____________. e.
BE CAREFUL! This formula is reactants minus products (NOT on formula chart). Calculating ΔH rxn from ΔH° f
uses products minus reactants (on formula chart).
Guided/Independent Practice
1. Using the bond energies from the reference chart above, estimate ΔH for the following reaction.
2. Does the reaction illustrate an endothermic (A) or exothermic (B) process?
H
2
(g) + Cl
2
(g) → 2HCl(g)
3. Estimate the carbon-fluorine bond energy given the remaining bond energies provided in the reference chart and the information provided in the equation below.
4. Does the reaction illustrate an endothermic (A) or exothermic (B) process?
(g) + F
2
(g)
→
(g) ΔH = -549kJ
5. Using the bond energies from the reference chart above, estimate ΔH for the following reaction.
6. Does the reaction illustrate an endothermic (A) or exothermic (B) process?
N
2
(g) + 3H
2
(g) → 2NH
3
(g)
7. Using the bond energies from the reference chart above, calculate ΔH for the reaction of methane with chlorine and fluorine to give Freon-12 (CF
2
Cl
2
). (You must use the calculated C-F bond energy from question 3).
8. Does the reaction illustrate an endothermic (A) or exothermic (B) process?
CH
4
(g) + 2Cl
2
(g) + 2F
2
(g) → CF
2
Cl
2
(g) + 2HF(g) + 2HCl(g)