chemistry - No Brain Too Small
advertisement
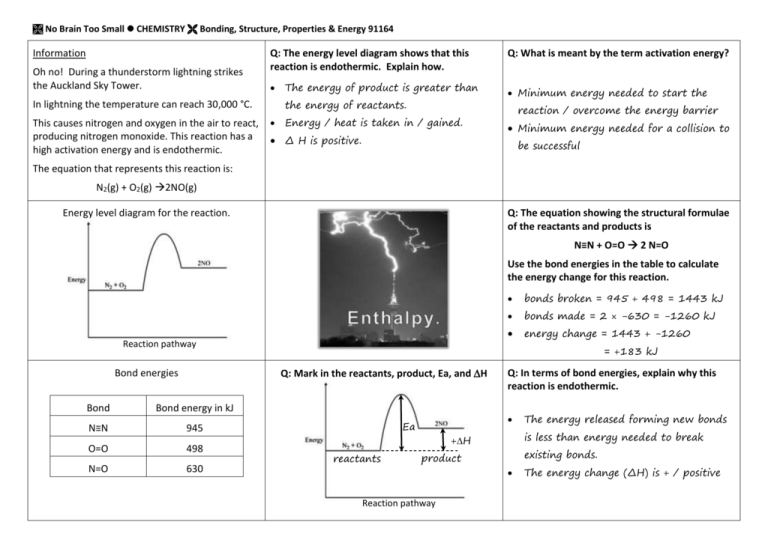
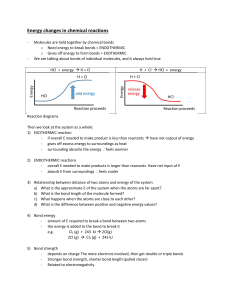
No Brain Too Small CHEMISTRY Bonding, Structure, Properties & Energy 91164 Information Oh no! During a thunderstorm lightning strikes the Auckland Sky Tower. Q: The energy level diagram shows that this reaction is endothermic. Explain how. the energy of reactants. In lightning the temperature can reach 30,000 °C. This causes nitrogen and oxygen in the air to react, producing nitrogen monoxide. This reaction has a high activation energy and is endothermic. The energy of product is greater than Energy / heat is taken in / gained. Δ H is positive. Q: What is meant by the term activation energy? Minimum energy needed to start the reaction / overcome the energy barrier Minimum energy needed for a collision to be successful The equation that represents this reaction is: N2(g) + O2(g) 2NO(g) Energy level diagram for the reaction. Q: The equation showing the structural formulae of the reactants and products is N≡N + O=O 2 N=O Use the bond energies in the table to calculate the energy change for this reaction. Reaction pathway Bond Bond energy in kJ N≡N 945 O=O 498 N=O 630 bonds broken = 945 + 498 = 1443 kJ bonds made = 2 × -630 = -1260 kJ energy change = 1443 + -1260 = +183 kJ Q: Mark in the reactants, product, Ea, and H Bond energies Ea reactants Q: In terms of bond energies, explain why this reaction is endothermic. is less than energy needed to break +H existing bonds. product Reaction pathway The energy released forming new bonds The energy change (ΔH) is + / positive No Brain Too Small CHEMISTRY Bonding, Structure, Properties & Energy 91164