Molecular Modeling Lab
advertisement

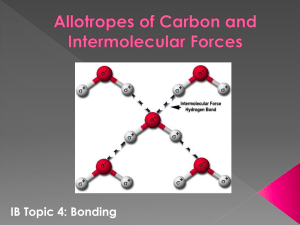
Lab - Covalent Bonds and Molecular Geometry March 4, 2010 OHS Chemistry To begin, complete the first two blank columns of the data table. For the column headed Bond Type use a periodic table to determine the difference in electronegativity (ΔEn) and record it in the column. Then write polar if any bond in the molecule is polar, otherwise write non-polar. Using the molecule sets, assemble each molecule of Set I. Your chemistry teacher will quiz you for understanding of the structures and check them off when correct. The model colors correspond to different elements: H—yellow; O—red; C—black; N—sky blue; Cl—green; Br—dark blue; F—orange; S —mystery. Under Shape indicate whether the molecule is linear, bent, pyramidal, or tetrahedral based on the central atom. Under Molecule Type indicate whether the molecule as a whole is polar or non-polar. Under 3-D drawing represent your molecule using lines, dashed lines and arrows to show the shape, including the positions of the unshared pairs. Repeat the procedure for Set II. Formula Electron-dot diagram Bond Type Set 1 e.g . CCl4 1. H2 2. F2 3. O2 4. N2 5. HBr 6. C2H2 7. CH3OH Shape Molecule Type 3-D drawing of Molecule Formula Electron-dot formula Bond Type Shape Molecule Type 3-D drawing of Molecule Set 2 8. H2O 9. CO2 10. H2S 11. NH3 12. CH4 13. H2O2 14. CH3Cl Questions: 1. Classify each of the following as: (1) ionic compound, (2) polar covalent molecule, (3) non-polar covalent molecule. a. Br2 e. SiO2 b. MgCl2 f. N2 c. CCl4 g. CH2I2 d. HI h. KBr 2. Both water and carbon dioxide are tri-atomic molecules (made up of three atoms). Explain why one is polar and the other is non-polar. Discuss and answer the following questions among members of your group for each molecular model. Be sure every member of your group understands about each molecule. I will be asking similar questions of you when I quiz you about the models. 1. What shape is the molecule? 2. What causes the molecule to be that shape? 3. Are the bonds polar or non-polar? 4. Is the molecule polar or non-polar? Explain why. 5. Which element is most electronegative in the molecule? 6. If the molecule is polar, where do the electrons spend most of their time? 7. If the molecule is polar, which end is positive/negative? 8. How would the molecule orient itself in space? 9. Which molecules have the strongest bonds? Discuss and answer the following questions among members of your group for each molecular model. Be sure every member of your group understands about each molecule. I will be asking similar questions of you when I quiz you about the models. 1. What shape is the molecule? 2. What causes the molecule to be that shape? 3. Are the bonds polar or non-polar? 4. Is the molecule polar or non-polar? Explain why. 5. Which element is most electronegative in the molecule? 6. If the molecule is polar, where do the electrons spend most of their time? 7. If the molecule is polar, which end is positive/negative? 8. How would the molecule orient itself in space? 9. Which molecules have the strongest bonds?