Performance in Delivering Research – Q2 (2015 – 2016)
advertisement

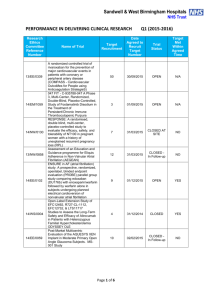
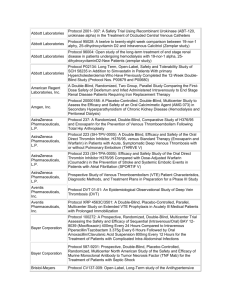
Sandwell & West Birmingham Hospitals NHS Trust PERFORMANCE IN DELIVERING CLINICAL RESEARCH Research Ethics Committee Reference Number 15/WA/0122 13/EE/0339 14/EM/1059 14/NW/0130 13/NW/0858 14/EE/0102 14/WS/0004 14/EE/0059 Name of Trial Clinical Investigation of the modified Rayner Monofocal Aspheric 600C (with axis marks) Intraocular Lens A randomized controlled trial of rivaroxaban for the prevention of major cardiovascular events in patients with coronary or peripheral artery disease (COMPASS - Cardiovascular OutcoMes for People using Anticoagulation StrategieS) 047 FIT - C-935788-047 A Phase 3, Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Study of Fostamatinib Disodium in the Treatment of Persistent/Chronic Immune Thrombocytopenic Purpura RESPONSE: A randomised, double blind, multi-center, placebo-controlled study to evaluate the efficacy, safety, and tolerability of NT100 in pregnant women with a history of unexplained recurrent pregnancy loss (RPL) Assessment of an Education and Guidance programme for Eliquis Adherence in Non-Valvular Atrial Fibrillation (AEGEAN) ENSURE in AF (atrial fibrillation) study. A prospective, randomized, openlabel, blinded endpoint evaluation (PROBE) parallel group study comparing edoxaban (DU176b) with enoxaparin/warfarin followed by warfarin alone in subjects undergoing planned electrical cardioversion of nonvalvular atrial fibrillation Open-Label Extension Study of EFC12492, R727-CL-1112, EFC12732, & LTS11717 Studies to Assess the Long-Term Safety and Efficacy of Alirocumab in Patients with Heterozygous Familial Hypercholesterolemia ODYSSEY OLE Post-Market Multicentric Evaluation of the AQUESYS XEN Implant in Moderate Primary Open Angle Glaucoma Subjects. MS-001 Study Q1 (2015-2016) Target Recruitment Date Agreed to Recruit Target Number Trial Status Target Met Within Agreed Time 20 31/11/2015 OPEN N/A 50 01/12/2015 OPEN N/A 3 01/12/2015 OPEN N/A 7 31/03/2015 CLOSED AT SITE NO 12 31/03/2015 CLOSED In Followup NO 9 01/12/2015 OPEN YES 4 31/12/2014 CLOSED YES 10 02/02/2015 CLOSED In Followup NO Page 1 of 6 14/EE/0102 14/SC/0032 14/SC/0100 13/LO/1320 13/NE/0269 13/EE/0241 14/WM/003 13/SC/0423 09/H0301/5 SPIRE 2: B1481038 Phase 3 multi-center, double-blind, randomized, Placebo-controlled, parallel group evaluation of the Efficacy, safety, and tolerability of pf-04950615, in reducing the Occurrence of major cardiovascular events in high risk Subjects Phase 3, Multi-Centre, DoubleBlind, Randomised, Placebocontrolled, Parallel Group Evaluation of the Efficacy, Safety and Tolerability of PF-04950615, in reducing the occurrence of major cardiovascular events in high risk subjects. SPIRE 1. Ocriplasmin for Vitreomacular Traction/Symptomatic Vitreomacular Adhesion A Phase III, Multi-National, MultiCenter, Randomized, Masked, Controlled, Safety and Efficacy Study of a Fluocinolone Acetonide Intravitreal (FAI) Insert in Subjects with Chronic Non-Infectious Uveitis Affecting the Posterior Segment of the Eye (pSivida) PR-30-5011-C - A Phase 3 Randomized Double-Blind Trial of Maintenance with Niraparib Versus Placebo in Patients with Platinum Sensitive Ovarian Cancer SIGNATURE. Secukinumab In patients with moderate to severe active, chronic plaque psoriasis who have failed on TNFα antaGoNists: A clinical Trial EvalUating Treatment REsults HYDRUS V: A Prospective, Multicentre, Randomised, Comparison of the Hydrus™ to the iStent™ for Lowering Intraocular Pressure in Primary Open Angle Glaucoma Spock - A dose finding study to assess the safety and efficacy of K-877 in patients with statincontrolled LDL-C but abnormal lipid levels THE CONTINUUM TRIAL. A Phase 3, Multi-centre, Randomised, Double-blind, Placebo-controlled, Parallel-group study of the Efficacy and Safety of Lenalidomide (Revlimid®) as Maintenance Therapy for Patients with B-Cell Chronic Lymphocytic Leukaemia following Second-line Therapy 2 20/12/2015 OPEN YES 5 20/12/2015 OPEN YES 6 31/03/2015 CLOSED YES 2 31/03/2015 CLOSED In Followup YES 2 30/09/2015 CLOSED In Followup YES 2 01/02/2015 CLOSED In Followup YES 10 28/04/2015 CLOSED In Followup NO 2 15/05/2014 CLOSED In Followup YES 5 31/08/2015 CLOSED NO Page 2 of 6 12/WM/0341 13/LO/1264 13/EM/0254 13/WM/0208 13/NE/0201 11/LO/1658 12/LO/0984 FOURIER. A Double-blind, Randomized, Placebo-controlled, Multicenter Study Assessing the Impact of Additional LDLCholesterol Reduction on Major Cardiovascular Events When AMG 145 is Used in Combination With Statin Therapy In Patients with Clinically Evident Cardiovascular Disease SOLO2. A Phase III, Randomised, Double Blind, Placebo Controlled, Multicentre Study of Olaparib Maintenance Monotherapy in Platinum Sensitive Relapsed BRCA Mutated Ovarian Cancer Patients who are in Complete or Partial Response Following Platinum based Chemotherapy PROMETHEUS . A 12-month, randomized, double-masked, sham-controlled, multicenter study to evaluate the efficacy and safety of 0.5mg ranibizumab intravitreal injections in patients with visual impairment due to vascular endothelial growth factor (VEGF) driven macular edema (ME) A Randomised, Double-blind, Placebo-controlled, Multiple Dose, Parallel, Multiple Dose-Level Study to Evaluate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Clinical Effect of AMG 557 in Systemic Lupus Erythematosus (SLE) Subjects with Active Lupus Arthritis SOLO 1. A Phase III, Randomised, Double Blind, Placebo Controlled, Multicentre Study of Olaparib Maintenance Monotherapy in Patients with BRCA Mutated Advanced (FIGO Stage III-IV) Ovarian Cancer following First Line Patinum Based Chemotherapy. LUMIGAN 056. A Multicenter, Double-masked, Randomized, Active-controlled, Parallel Study of the Safety and Efficacy of Oncedaily Bimatoprost Preservativefree Ophthalmic Solution Compared to Twice-daily Timolol Ophthalmic Solution in Paediatric Patients With Glaucoma A Randomised, Open-Label, Phase 2 Study of the IDO Inhibitor INCB024360 (I) Versus Tamoxifen (C) for Subjects with Biochemical-recurrent-only Epithelial ovarian cancer, Primary Peritoneal Carcinoma or Fallopian Tube Cancer (P) Following Complete Remission with Firstline Chemotherapy. 30 31/03/2015 CLOSED In Followup YES 6 08/01/2015 CLOSED In Followup NO 2 06/08/2014 CLOSED In Followup YES 3 30/05/2015 CLOSED NO 4 08/01/2015 CLOSED NO 2 01/09/2014 CLOSED In Followup NO 4 01/08/2014 CLOSED NO Page 3 of 6 13/NW/0363 13/LO/0528 12/WS/0300 13/WM/0046 12/WS/0184 13/LO/0049 11/EM/0074 13/EM/0017 OSLER 2: A Multicenter Openlabel Extension (OLE) Study to Assess the Long-term Safety and Efficacy of AMG 145 PIONEER-AF. An Open-label, Randomized, Controlled, Multicenter Study ExplorIng Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention ODYSSEY OUTCOMES: A Randomized, Double-Blind, Placebo-Controlled, ParallelGroup Study to Evaluate the Effect of SAR236553/REGN727 on the Occurrence of Cardiovascular Events in Patients Who Have Recently Experienced an Acute Coronary Syndrome HYDRUS III. A Prospective, Multicentre, Randomised Comparison of the Hydrustm Aqueous Implant to the iStent for lowering Intraocular Pressure in Glaucoma Subjects Undergoing Cataract Surgery Multi-Center, Open-Label, Randomized Study of Anti-CCR4 Monoclonal Antibody KW-0761 versus Investigator’s Choice in Subjects with Previously Treated Adult T-cell Leukemia-Lymphoma (ATL) REPARO. An 8-week phase I/II, multicenter, randomized, doublemasked, vehicle controlled parallel group study with a 48 or 56 week follow-up period to evaluate the safety and efficacy of two doses (10 µg/ml and 20 µg/ml) of recombinant human nerve growth factor eye drops solution versus vehicle in patients with Stage 2 and 3 of Neurotrophic Keratitis LUMIGAN 054. A 2-year, multicenter, double-masked, randomized, parallel study of the safety of LUMIGAN® 0.1 mg/mL compared with LUMIGAN® 0.3 mg/mL in patients with glaucoma or ocular hypertension A 3-Month, Multi-Center, DoubleMasked Safety and Efficacy Study of Travoprost Ophthalmic Solution, 0.004% Compared to Timolol (0.5% or 0.25%) in Pediatric Glaucoma Patients 2 12/05/2014 CLOSED In Followup NO 8 31/08/2015 OPEN YES 20 31/12/2015 OPEN N/A 18 28/04/2015 CLOSED In Followup NO 3 01/05/2015 CLOSED In Followup YES 2 01/06/2015 CLOSED In Followup NO 12 31/10/2014 CLOSED In Followup NO 2 01/08/2013 CLOSED In Followup YES Page 4 of 6 12/EE/0533 12/EE/0532 12/NW/0670 12/WM/0341 12/LO/1650 12/WM/0348 12/WM/0290 ODYSSEY Options II. A Randomized, Double-Blind Study of the Efficacy and Safety of REGN727 Added-on to Rosuvastatin versus Ezetimibe Added-on to Rosuvastatin versus Rosuvastatin Dose Increase in Patients Who are Not Controlled on Rosuvastatin (Regeneron R727-CL-1118) ODYSSEY OPTIONS I. A Randomised, Double-Blind Study of the Efficacy and Safety of REGN727 Added-on to Atovastatin versus Ezetimibe Added-on to Atorvastatin versus Atorvastatin Dose Increase versus Switch to Rosuvastatin in Patients Who are Not Controlled on Atorvastatin (Regeneron R727CL-1110) A Randomized, Double-blind, Placebo-controlled Study to Evaluate the Safety, Tolerability, and Efficacy of AMG 181 in Subjects with Moderate to Severe Crohn’s Disease (Protocol 20110232) TRAFFIC CD FOURIER. A Double-blind, Randomized, Placebo-controlled, Multicenter Study Assessing the Impact of Additional LDLCholesterol Reduction on Major Cardiovascular Events When AMG 145 is Used in Combination With Statin Therapy In Patients with Clinically Evident Cardiovascular Disease EYEGUARD-B: A randomized, double-masked, placebocontrolled study of the efficacy of gevokizumab in the treatment of patients with Behçet’s Disease uveitis. The RELIEF Study. Polycythemia Vera Symptom Study Evaluating Ruxolitinib Versus Hydroxyurea in a Randomized, Multicenter, Double-Blind, Double-Dummy, Phase 3 Efficacy and Safety Study of Patient Reported Outcomes A double, randomised, multicentre study to evaluate safety and efficacy of AMG 145, compared with ezetimibe, in hypercholesterolemic subjects unable to tolerate an effective dose of a HMG-CoA reductase inhibitor 20110116 (GAUSS 2) 3 01/08/2013 CLOSED In Followup NO 3 09/08/2013 CLOSED NO 5 30/11/2014 CLOSED In Followup NO 30 31/03/2015 CLOSED In Followup YES 2 01/09/2014 CLOSED NO 1 15.11.2013 CLOSED In Followup YES 6 23/07/2013 CLOSED In Followup NO Page 5 of 6 12/LO/0482 12/NW/0014 10/H0718/93 11/LO/1619 PERUSE. A multicenter, openlabel, single-arm study of pertuzumab in combination with trastuzumab and a taxane in first line treatment of patients with HER2-positive advanced (metastatic or locally recurrent) breast cancer. FOCUS FH. A Phase 3, Randomized, Double-Blind, Placebo-Controlled, ParallelGroup Study to Assess the Safety and Efficacy of Two Different Regimens of Mipomersen in Patients with Familial Hypercholesterolemia and Inadequately Controlled LowDensity-Lipoprotein Cholesterol (MIP038) ALLOS PDX-017. A Multi-center, Randomized, Phase 3 Study of Sequential Pralatrexate Versus Observation in Patients with Previously Undiagnosed Peripheral T-cell Lymphoma Who Have Achieved an Objective Response Following Initial Treatment with CHOP-based Chemotherapy ALTERNATIVE. Comparing safety & efficacy of AI/lapatanib/trastuzumab in HR+HER2 MBC 4 30/11/2013 CLOSED In Followup NO 6 22/11/2013 CLOSED In Followup NO 7 30/08/2013 CLOSED In Followup NO 5 02/01/2015 CLOSED NO Page 6 of 6