ONGOING CLINICAL TRIALS
advertisement

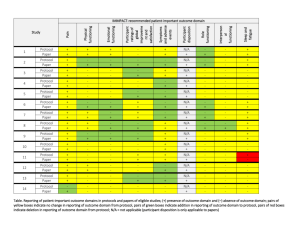
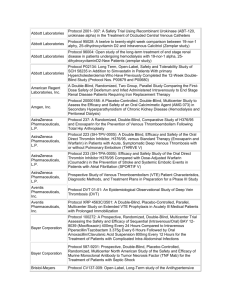
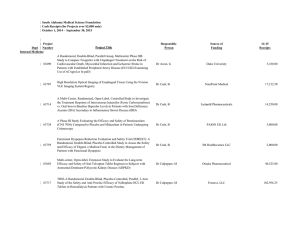
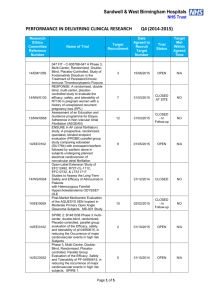
ONGOING CLINICAL TRIALS University of Vermont Presented by: Rup Tandan, MD, FRCP TRIAL OF HIGH FAT / HIGH CALORIE DIET IN ALS PI: Rup Tandan, MD Supported By: Muscular Dystrophy Association Objectives: To compare the safety and tolerability in three subject groups randomized to high fat/high calorie, high calorie or control diets To measure biomarkers of body composition and lipid metabolism before and during diet intervention To examine preliminary effects of the three diets on measures of disease progression Enrollment closes 10/31/12 ALS RESEARCH GROUP DNA BANKING STUDY PI: Rup Tandan, MD Supported By: The National Institutes of Health (NIH), the Muscular Dystrophy Association, and the Amyotrophic Lateral Sclerosis Association (ALSA) Objectives: To look at genetic factors that may contribute to the development of ALS and which may improve the genetic diagnosis of ALS and related Motor Neuron Diseases. A MULTI-CENTER, SINGLE BLIND, RANDOMIZED STUDY COMPARING THYMECTOMY TO NON THYMECTOMY IN NON-THYMOMATOUS MYASTHENIA GRAVIS (MG) PATIENTS RECEIVING PREDNISONE PI: Rup Tandan, MD Supported By: The National Institutes of Neurological Disorders & Stroke (NINDS) Objectives: The main purpose of this research is to determine whether thymectomy has a positive influence on the management of patients with myasthenia gravis who are taking prednisone and who do not have a thymoma. The project's ancillary Biomarkers study will evaluate genetic factors and gene and protein expression in the blood and thymus (in patients undergoing thymectomy) which may influence MG. Enrollment closes in November 2012 CONTACT INFORMATION for Dr. Tandan’s Studies Rup Tandan, MD - Site PI (802) 847-4589 Rup.Tandan@vtmednet.org Study Coordinator for Nutrition Study and Thymectomy Trial (802) 656-4582 Shannon.Lucy@med.uvm.edu A MULTICENTER, DOUBLE-BLIND, PARALLEL GROUP, PLACEBO CONTROLLED STUDY OF CREATINE IN SUBJECTS WITH TREATED PARKINSON’S DISEASE (PD). LONG-TERM STUDY – 1 (LS-1) PI: Robert Hamill, MD Supported By: NIH, National Institute of Neurological Disorders and Stroke (NINDS) Objective: To determine if there is a slowing of clinical decline in PD patients defined by a combination of cognitive, physical, and quality of life measures. NET-PD LS-1 1,720 subjects from approximately 52 US and Canadian sites will be equally randomized to the study arms. Main Inclusion Criteria Subject is willing and able to give informed consent and commit to long-term follow-up PD within 5 years of diagnosis Treated/responsive to dopaminergic therapy (dopamine agonists or levodopa) for at least 90 days, but not more than 2 years Enrollment was closed as of May 2011. There are 27 active patients at our site. A MULTICENTER, DOUBLE-BLIND, PLACEBO CONTROLLED, PHASE II STUDY OF PIOGLITAZONE IN EARLY PARKINSON’S DISEASE (PD). (FS-ZONE) PI: James Boyd, MD Supported By: NIH, National Institute of Neurological Disorders and Stroke (NINDS) Objective: To assess the impact of pioglitazone on the progression of PD in order to determine whether it is futile to proceed with further study of this agent. The secondary objectives of the study are to collect additional efficacy and safety/tolerability data to be used in planning a subsequent Phase III trial of pioglitazone in early, treated PD. NET-PD FS-ZONE Approximately 216 subjects from approximately 43 sites in the US will be enrolled in this study to assess the impact of two doses of pioglitazone on the clinical decline of PD. Main Inclusion Criteria Subject is willing and able to give informed consent and commit to 44 weeks of follow-up Subjects who are on stable dose of rasagiline 1 mg/day or selegiline 10 mg/day for at least 8 weeks but no more than 8 months CONTACT INFORMATION Robert Hamill, MD - Site PI (802) 847-4589 Robert.Hamill@vtmednet.org Study Coordinator: (802) 656-3878 Emily.houston@uvm.edu EPILEPSY CLINICAL TRIALS CLOSED TO FURTHER ENROLLMENT An Open-Label Extension Phase of the Double-blind, Placebo-controlled, Dose-escalation, Parallel-group Study of E2007 (perampanel) as an Adjunctive Therapy in Patients with Refractory Partial Seizures An open-label, multinational, multicenter, follow-up study to evaluate the long-term safety and efficacy of brivaracetam, used at a flexible dose up to a maximum of 150 mg/day, in subjects aged 16 years or older suffering from epilepsy. THE MS CENTER OF NORTHERN NEW ENGLAND AT UVM/FLETCHER ALLEN Angela Applebee, MD – Director Andrew Solomon, MD Sandra McGrath, RN FNP Patty Krusinski, CCRC, Study Coordinator AnneMarie Savage, RN, Research Nurse Jane Low, MPA, Study Coordinator ONGOING CLINICAL TRIALS IN MS CLOSED TO FURTHER ENROLLMENT Novartis 2306 A double-blind, randomized, multicenter, placebo-controlled, parallel-group study evaluating safety and efficacy of 0.5mg FTY720 (fingolimod) in patients with PPMS Novartis 2309 A DB, PC RCT to examine safety and efficacy of 2 doses of FTY720 (fingolimod) capsules in RRMS over 24 months. Biogen Idec – Daclizumab Study in Relapsing-Remitting MS To determine the efficacy and safety of daclizumab high yield process (DAC HYP) versus Avonex® (Interferon β-1a) in patients with relapsing-remitting MS. ADDITIONAL CLINICAL TRIALS IN MS CLOSED TO FURTHER ENROLLMENT (Continued) Extension Study AC-058B202 Extension study to AC-058B201 to study long-term safety, tolerability and efficacy of 3 doses of ACT-128800 in RRMS EFC6260 – Sanofi-Aventis International, multi-center, randomized, double-blind, placebocontrolled, parallel-group study to evaluate efficacy and safety of teriflunomide in patients with a first clinical episode suggestive of MS. ONGOING CLINICAL TRIALS IN MS OPEN TO ENROLLMENT Stratify – 2 Biogen JCV Anitbody program in patients with RRMS receiving or considering treatment with Tysabri Genentech/Roche Ocrelizumab study in Relapsing MS A multicenter, randomized trial to evaluate the safety and efficacy of ocrelizumab in comparision to interferon beta-1a (Rebif®) in patients with relapsing MS Opexa Therapeutics, Inc. In Secondary Progressive MS A multicenter, double-blind trial to evaluate the safety and effectiveness of an investigational T-cell product when compared to placebo in patients with secondary progressive MS STROKE/NEUROCRITICAL CARE IRIS (Insulin Resistance Intervention after Stroke) PI: Mark Gorman, MD NINDS-funded multicenter randomized clinical secondary prevention trial Looks at reduction of recurrent stroke, MI and death in insulin resistant stroke/TIA patients Intervention is pioglitazone 45 mg (insulin sensitizer/PPAR- agonist) vs. placebo Follow-up 3-5 years IRIS STUDY Contact Information Study Coordinator: Catherine.Gregory@med.uvm.edu 802-656-8993 Principal Investigator: mgorman1@uvm.edu ABBOTT PARKINSON’S STUDY PI: James Boyd, MD Study of levodopa/carbidopa intestinal gel vs. oral levodopa/carbidopa For pts with advanced Parkinson’s, who experience motor fluctuations and 3 or more hours of “off” time daily, despite optimized levodopa treatment. Closed to enrollment in July 2011. Pts who have had DBS or other surgery for Parkinson’s are not eligible ABBOTT PARKINSON’S STUDY (Continued) Levodopa/carbidopa intestinal gel is an approved therapy in Europe known as Duodopa. Under testing in U.S. for FDA approval. Medication delivered through a PEG with jejunal extension tube, by continuous infusion pump pts wear 16 hrs per day. Intended to stabilize dopamine levels and minimize motor fluctuations. ABBOTT PARKINSON’S STUDY Contact Information Study Coordinator: Emily.Houston@uvm.edu 802-656-3878 Principal Investigator: James.Boyd@uvm.edu