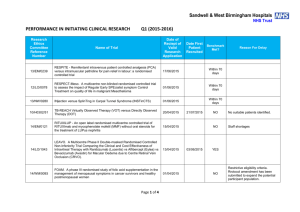
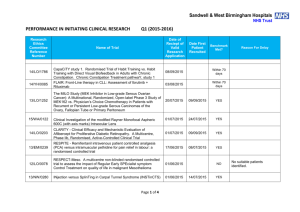
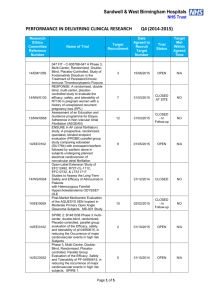
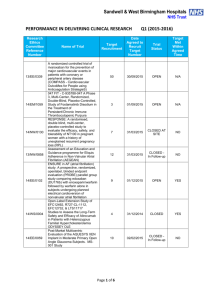
Performance in Initiating Clinical Research – Q4 (2014
advertisement
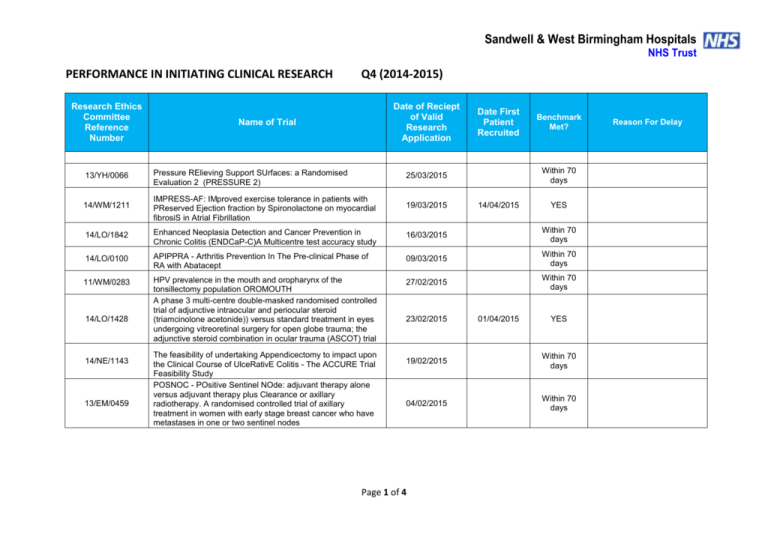
Sandwell & West Birmingham Hospitals NHS Trust PERFORMANCE IN INITIATING CLINICAL RESEARCH Research Ethics Committee Reference Number Q4 (2014-2015) Date of Reciept of Valid Research Application Name of Trial Date First Patient Recruited Benchmark Met? Within 70 days 13/YH/0066 Pressure RElieving Support SUrfaces: a Randomised Evaluation 2 (PRESSURE 2) 25/03/2015 14/WM/1211 IMPRESS-AF: IMproved exercise tolerance in patients with PReserved Ejection fraction by Spironolactone on myocardial fibrosiS in Atrial Fibrillation 19/03/2015 14/LO/1842 Enhanced Neoplasia Detection and Cancer Prevention in Chronic Colitis (ENDCaP-C)A Multicentre test accuracy study 16/03/2015 Within 70 days 14/LO/0100 APIPPRA - Arthritis Prevention In The Pre-clinical Phase of RA with Abatacept 09/03/2015 Within 70 days 11/WM/0283 HPV prevalence in the mouth and oropharynx of the tonsillectomy population OROMOUTH A phase 3 multi-centre double-masked randomised controlled trial of adjunctive intraocular and periocular steroid (triamcinolone acetonide)) versus standard treatment in eyes undergoing vitreoretinal surgery for open globe trauma; the adjunctive steroid combination in ocular trauma (ASCOT) trial 27/02/2015 Within 70 days 14/LO/1428 14/NE/1143 13/EM/0459 The feasibility of undertaking Appendicectomy to impact upon the Clinical Course of UlceRativE Colitis - The ACCURE Trial Feasibility Study POSNOC - POsitive Sentinel NOde: adjuvant therapy alone versus adjuvant therapy plus Clearance or axillary radiotherapy. A randomised controlled trial of axillary treatment in women with early stage breast cancer who have metastases in one or two sentinel nodes 23/02/2015 14/04/2015 01/04/2015 YES YES 19/02/2015 Within 70 days 04/02/2015 Within 70 days Page 1 of 4 Reason For Delay 14/NW/1351 Rehabilitation Enablement in Chronic Heart Failure (REACH HF). A multicentre parallel group randomised controlled trial with parallel economic and process evaluation to assess the clinical effectiveness and cost-effectiveness of the REACH HF manual for patients and caregivers. 23/01/2015 14/WM/0057 Multicentre randomised controlled trial to compare the clinical and costeffectiveness of a ‘vein bypass first’ with a ‘best endovascular first’ revascularisation strategy for severe limb ischaemia due to infrapopliteal arterial disease: Bypass vs. Angioplasty in Severe Ischaemia of the Leg. (BASIL- 2) 07/01/2015 13/EE/0339 A randomized controlled trial of rivaroxaban for the prevention of major cardiovascular events in patients with coronary or peripheral artery disease (COMPASS - Cardiovascular OutcoMes for People using Anticoagulation StrategieS) 07/01/2015 13/LO/0145 13/LO0651 A multicentre phase III randomised controlled single masked clinical trial to test the clinical efficacy of LightMasks at preventing dark adaptation in the treatment of early diabetic macular oedema (CLEOPATRA) A randomised controlled trial of a biomarker-based exclusion of VAP to improve antibiotic stewardship. VAPrapid-2 13/SC/0111 10/02/2015 YES NO 03/03/2015 YES NO Grren light for study to open not given until 10/02/015. First participant recruited within three weeks of green light 08/12/2014 NO No suitable patients identified. 05/12/2014 NO Site still not activated. SIV now arranged for last week in April 2015 17/12/2014 04/03/2015 FOCUS4. Molecular selection of therapy in colorectal cancer 14/WM/0159 14/EM/1059 14/NW/0327 14/NW/0130 12/SS/0138 Small patient population Safety of Nasal Influenza Immunisation in Egg Allergic Children - The SNIFFLE 2 Study 047 FIT - C-935788-047 A Phase 3, Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Study of Fostamatinib Disodium in the Treatment of Persistent/Chronic Immune Thrombocytopenic Purpura 03/11/2014 Effects of Glaucoma Surgery on Corneal Endothelial Cells GICES RESPONSE: A randomised, double blind, multi-center, placebo-controlled study to evaluate the efficacy, safety, and tolerability of NT100 in pregnant women with a history of unexplained recurrent pregnancy loss (RPL) 10/10/2014 REstart or STop Antithrombotics Randomised Trial (RESTART) 30/09/2014 17/12/2014 30/10/2014 17/02/2015 07/10/2014 Page 2 of 4 28/11/2014 YES NO Eligibility criteria prohibitive requiring very specific disease criteria. NO Staffing issues at site NO Study closed at site due to resource issues YES 13/EM/0395 Treatment of Advanced Glaucoma Study (TAGS) 01/10/2014 NO NO 13/EM/0398 Phase II clinical trial investigating the use of epigallocatechin3-gallate (Veregen) in the treatment of vulval intraepithelial neoplasia. EPIVIN trial (v 1.0) 17/09/2014 13/10/2014 YES Assessment of an Education and Guidance programme for Eliquis Adherence in Non-Valvular Atrial Fibrillation (AEGEAN) ENSURE in AF (atrial fibrillation) study. A prospective, randomized, openlabel, blinded endpoint evaluation (PROBE) parallel group study comparing edoxaban (DU176b) with enoxaparin/warfarin followed by warfarin alone in subjects undergoing planned electrical cardioversion of nonvalvular atrial fibrillation 04/09/2014 22/10/2014 YES 04/09/2014 20/10/2014 YES 12/WM/00335 A pilot study for developing and evaluating a care pathway for cognitive problems after stroke (OCS-care) 28/07/2014 28/08/2014 YES 14/WS/0004 Open-Label Extension Study of EFC12492, R727-CL-1112, EFC12732, & LTS11717 Studies to Assess the Long-Term Safety and Efficacy of Alirocumab in Patients with Heterozygous Familial Hypercholesterolemia ODYSSEY OLE 22/07/2014 25/09/2014 YES 14/WM/0083 A Phase III Trial of Surgery versus Active Monitoring for Low Risk Ductal Carcinoma in Situ. The Low Risk DCIS Trial (LORIS). 23/07/2014 14/08/2014 YES 14/EE/0059 Post-Market Multicentric Evaluation of the AQUESYS XEN Implant in Moderate Primary Open Angle Glaucoma Subjects. MS-001 Study 18/07/2014 10/09/2014 YES 13/NW/0858 14/EE/0102 14/EE/0102 13/EM/0427 12/LO/1168 SPIRE 2: B1481038 Phase 3 multi-center, double-blind, randomized, Placebo-controlled, parallel group evaluation of the Efficacy, safety, and tolerability of pf-04950615, in reducing the Occurrence of major cardiovascular events in high risk Subjects MiQuit trial: Tailored text messages for pregnant women v1 Subcutaneous Bortezomib, Cyclophosphamide and Rituximab (BCR) versus Fludarabine, Cyclophosphamide and Rituximab (FCR) for initial therapy of Waldenstrõm macroglobulinaemia: a randomised phase II study. (R2W) 10/07/2014 NO 03/07/2014 29/07/2014 YES 25/06/2014 04/11/2014 NO Page 3 of 4 No suitable patients identified. Sponsor delays: Green light to start the study not given until 18/12/2014. Eligibility criteria prohibitive requiring very specific disease criteria. Small patient population 14/SC/0032 Phase 3, Multi-Centre, Double-Blind, Randomised, Placebocontrolled, Parallel Group Evaluation of the Efficacy, Safety and Tolerability of PF-04950615, in reducing the occurrence of major cardiovascular events in high risk subjects. SPIRE 1. 19/06/2014 No NO Sponsor delays: Green light to start the study not given until 18/12/2014. Eligibility criteria prohibitive requiring very specific disease criteria. 12/LO/1319 A Phase 2 Study for Older Adults with Acute Lymphoblastic Leukaemia UKALL 60+ 11/06/2014 No NO Small patient population 14/EE/0032 Pilot study: Finger prick fresh blood for treatment of chronic corneal ulcers, persistent epithelial defects and dry eyes. 03/06/2014 08/08/2014 YES TARDIS - Safety and efficacy of intensive versus guideline antiplatelet therapy in high risk patients with recent ischaemic stroke or transient ischaemic attack: a randomised controlled trial 12/05/2014 No NO Ocriplasmin for Vitreomacular Traction/Symptomatic Vitreomacular Adhesion 09/05/2014 14/05/2014 YES 07/04/2014 19/05/2014 YES 08/H1102/112 14/SC/0100 13/LO/1320 A Phase III, Multi-National, Multi-Center, Randomized, Masked, Controlled, Safety and Efficacy Study of a Fluocinolone Acetonide Intravitreal (FAI) Insert in Subjects with Chronic Non-Infectious Uveitis Affecting the Posterior Segment of the Eye (pSivida) Page 4 of 4 Study closed at site due to lack of suitable patients presenting