Oscar Lin, MD
advertisement

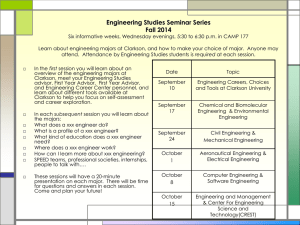
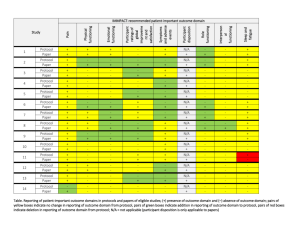
Oscar Lin, MD 145 Calverton Road Calverton Park, MO 63135 573-808-2910 lino3872@gmail.com Curriculum Vitae Medical Training 2004 Board Certification in Radiology 2003-2004 Radiology Chief Resident University of Missouri-Columbia 1999-2003 Radiology Residency Univeristy of Missouri-Columbia Education 1995-1999 Doctor of Medicine University of Missouri-Columbia 1990-1994 Bachelor of Science, Biology, Cum Laude Univeristy of Missouri-Columbia 1990-1994 Bachelor of Fine Arts, Cum Laude University of Missouri-Columbia 1986-1990 Moberly Senior High School, Valedictorian Licenses and Certifications Missouri State Medical License Illinois State Medical License Board Certified Radiologist Employment Radiologic Physicians LTD HealthCare Research 637 Dunn Road, Suite 135 Hazelwood, MO 63031 July 2004-present August 2009-present Hospital Affiliations Saint Anthnoy’s Health Center #1 St. Anthony’s Way Alton, IL 62002 Jersey Community Hospital 400 Maple Summit Road Jerseyville, IL 62025 Language Proficiency Fluent in Taiwanese Basic Spanish Professional Affiliations American Medical Association Society of Radiologists in Ultrasound Research Experience 1. A 12-Month, Open-Label Study to Evaluate the Long-Term Safety of XXX at 1590mg Every 12 Hours in Patients Who Require Opioid Treatment for an Extended Period of Time. 2. A 12-Week, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate The Efficacy and Safety of XXX at 15-90 mg Every 12 Hours for Relief of Moderate to Severe Pain in Patients With Osteoarthritis or Low Back Pain Who Require Opioid Treatment for an Extended Period of Time. 3. A Phase 2A, Randomized, Blinded, Placebo and Active Controlled, 2 Period Crossover Study to Assess the Analgesic Efficacy, Safety, and Tolerability of XXX in Subjects with Postherpectic Neuralgia. 4. A 4 Week, Double-Blind, Placebo-Controlled, Randomized, MultiCenter Study Evaluating the Safety and Efficacy of XXX in Subjects with Bladder Pain Syndrome. 5. The Efficacy and Safety of XXX in the Treatment of Osteoarthrits of the Knee: Pivotal Study 1. 6. A Phase 2a, Randomized, Double-blind, Placebo- and Active-Controlled, ParallelGroup, Multicenter Study Evaluating the Analgesic Efficacy and Safety of XXX And XXX in Subjects With Moderate to Severe Pain Due to Osteoarthrtis of the Knee. 7. A Multicenter, Randomized, Active-Control, Phase3B Study to Evaluate the Cardiovascular Safety of XXX and XXX in Subjects With Gout and Cardiovascular Comorbidities. 8. A Phase 3, Open-Label Period Followed by a Randomized, Double-Blind, Placebo-Controlled Study of the Analgesic Efficacy of XXX Compared to Placebo in Subjects with Chronic Low Back Pain.