Ginger`s 2015 Resume - iSearch
advertisement

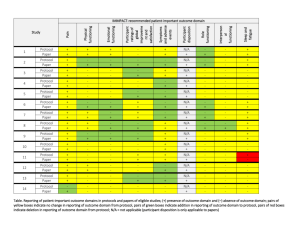
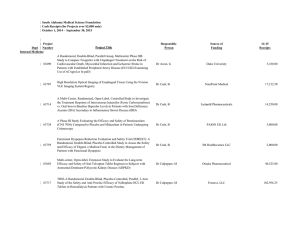
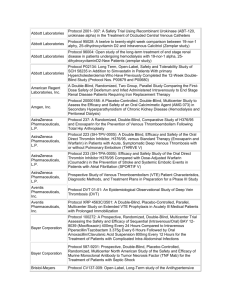
Ginger S. Rappley 5029 Iron Horse Way Ave Maria, FL 34142 gingerrappley@icloud.com (715) 490-5329 ____________________________________________________________________ __________________________________________________________ Clinical Research Management/Regulatory Science Clinical Research Management student who promotes a cohesive environment and has experience in supervisory and management positions. Proven leadership, ethics and excellent customer service and patient care skills. Skills/Capabilities · Direct day-to-day operations and decision-making, including managing resources, personnel, inventory, and workflow. · Analyze patient satisfaction data and quality assurance reports to identify improvement opportunities · Improve performance by measuring clinical effectiveness, monitoring safety metrics, and performing life safety and environment of care audits. · Manage high-level financial and analytical analyses, including budgeting, forecasting and reporting. · Prioritize areas of organizational risk, incorporating them into quality improvement plans. · Experience in QuickBooks, Microsoft Office, SAP, QAD, Clinical Conductor, RAVE, Medidata Certified. · Customer correspondence and inventory management. · Clinical experience (RN & CNA programs) in both nursing home and hospital care, including CNA certification in both. · Planning, implementing, and maintaining data recording systems in support and accordance with research protocols. · Ensuring scientific integrity of data and protecting the rights, safety, and well-being of patients enrolled in clinical trials. · Technical duties: phlebotomy along with specimen processing, EKG, and vital signs; additionally, calibration and maintenance of study equipment · Scheduling for patient visits in accordance with research protocol and serving as liaison for arranging audit visits to the site · Medical records review: read, examine, identify, and scan treatment records, progress notes, and other documentation relative to specific medical conditions to verify inclusion/exclusion criteria as determined by research protocols · Preparing periodic and/or ad hoc reports on research activities Education Arizona State University – Phoenix, AZ Master of Science - Clinical Research Management (Regulatory Science) Expected Graduation: December 2016 Franklin University – Columbus, OH Bachelor of Science – Allied Healthcare Management December 2014 Employment Experience Southwest Florida Research, 2014-present, Clinical Research Coordinator. Coordinates and administers clinical research trials. Assist in project planning, ensures that pre-established work scope, study protocol and regulatory requirements are followed. Recruits and coordinates research subjects. Performs diverse administrative duties requiring analysis, sound judgment and a high level of knowledge of study specific protocols. Maintains complex recordkeeping systems. Studies worked on: 1 | Rappley ACCELERATE A Phase 3, multicenter, randomized, parallel group, double-blind, placebo-controlled, event-driven study assessing clinical effects of cholesterol ester transfer protein (CETP) inhibition with Evacetrapbid in patients at a high risk for vascular outcomes. (Lilly) CAMELLIA A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Effect of Long Term Treatment with BELVIQ (lorcaserin HCL) on the Incidence of Major Adverse Cardiovascular Events and Conversion to Type 2 Diabetes mellitus on Obese and Overweight Subjects with Cardiovascular Disease or Multiple Cardiovascular Risk Factors (Eisai) CIRT A randomized, double-blind, placebo-controlled, event-driven trial of weekly low-dose methotrexate (LDM) in the prevention of recurrent cardiovascular events among stable post-myocardial infarction patients with type 2 diabetes or metabolic syndrome (National Heart Lung and Blood Institute) DECLARE Dapagliflozin Effect of Cardiovascular Events. A multicenter, randomized, double-blind, placebo-controlled trial to evaluate the effect of Dapagliflozin 10 mg once daily on the incidence of Cardiovascular Death, Myocardial Infarction or Ischemic Stroke in patients with Type 2 Diabetes. (Bristol-Myers Squibb) PEGASUS (TIMI-54) A Trial to Assess the Prevention of Thrombotic Events with Ticagrelor compared to a Placebo on Patients on ASA (Aspirin) Therapy and a Recent History of Myocardial Infarction (AstraZeneca AB) REVEAL (TIMI-55) A Large-scale, Randomized Placebo-controlled Trial of the Clinical Effects of Anacetrapib Among People With Established Vascular Disease (Merck) SOCRATES (PRESERVED & REDUCED) A randomized parallel-group, placebo-controlled, double-blind, multicenter dose finding phase II trial exploring the pharmacodynamic effects, safety and tolerability, and pharmacokinetics of four dose regimens of the oral sGC stimulator BAY 1021189 over 12 weeks in patients with worsening heart failure and preserved ejection fraction (HFpEF) - SOluble Guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF/REDUCED EF (Bayer) SPIRE 1 & 2 (PCSK9) Phase 3 multi-center, double-blind, randomized, placebo-controlled, parallel group evaluation of the efficacy, safety, and tolerability of Bococizumab, in reducing the occurrence of major cardiovascular events in high risk subjects. Arthrex, 2014-2014, Inventory Control Clerk. Ensure that throughput, workflow and production plan goals are accomplished by kitting work orders accurately and in a timely manner. Assist Kitting Department by conducting training, participating in process improvement and problem solving. Support programs, policies and procedures in order to help the growth of Arthrex Manufacturing, Inc. as a world-class medical device manufacturer. Precise data entry to ensure accurate information. United States Postal Service, 2006-2014, Rural Mail Carrier Associate. Preparation and delivery of mail to residential/business customers in a rural capacity. Professional Highlights · Certified Nursing Assistant, Acute & Long Term Care, State of Wisconsin – 2001-2003 · “Seek Perfection, Catch Excellence” Award – Annual Certificate of Recognition for Outstanding Employees recognized for their hard work and dedication. - Mark Rask, Manager of Post Office Operations, April 2007 · Scientia Pietas Veritas, Healthcare Honor Society – Franklin University, 2014 · Medidata Rave Certified Clinical Research Coordinator – 2014 · National Institutes of Health Office of Extramural Research Certification – 2014 · Good Clinical Practices, NIDA Clinical Trials Network – 2014 · Dean’s List, President’s List, Franklin University, 2011 – 2014 · Certified Phlebotomist, Clinical Solutions, 2015 Activities 2 | Rappley · · · · · · 3 | Rappley Religious Education Instructor, Nativity of our Lord Parish 2005-2010 Ministry of Catechesis Certification, Diocese of Superior, 2007 Lay Ministry Outreach Formation Program, Nativity of our Lord Parish, 2006-2008 Baptism Class Instructor, Nativity of our Lord Parish, 2009-2010 Committee of Education, Nativity Catholic School, 2008-2010 Member, Southwest Florida Guild of the Catholic Medical Association, 2013 - present