Experiment-Hardness
advertisement
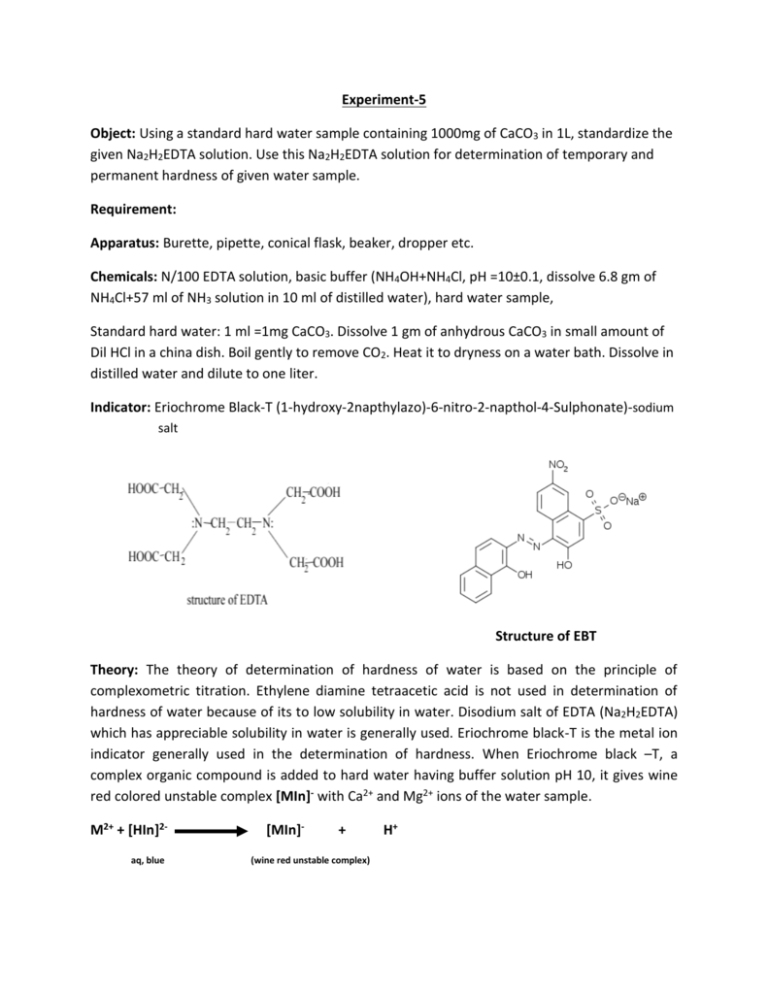
Experiment-5 Object: Using a standard hard water sample containing 1000mg of CaCO3 in 1L, standardize the given Na2H2EDTA solution. Use this Na2H2EDTA solution for determination of temporary and permanent hardness of given water sample. Requirement: Apparatus: Burette, pipette, conical flask, beaker, dropper etc. Chemicals: N/100 EDTA solution, basic buffer (NH4OH+NH4Cl, pH =10±0.1, dissolve 6.8 gm of NH4Cl+57 ml of NH3 solution in 10 ml of distilled water), hard water sample, Standard hard water: 1 ml =1mg CaCO3. Dissolve 1 gm of anhydrous CaCO3 in small amount of Dil HCl in a china dish. Boil gently to remove CO2. Heat it to dryness on a water bath. Dissolve in distilled water and dilute to one liter. Indicator: Eriochrome Black-T (1-hydroxy-2napthylazo)-6-nitro-2-napthol-4-Sulphonate)-sodium salt Structure of EBT Theory: The theory of determination of hardness of water is based on the principle of complexometric titration. Ethylene diamine tetraacetic acid is not used in determination of hardness of water because of its to low solubility in water. Disodium salt of EDTA (Na2H2EDTA) which has appreciable solubility in water is generally used. Eriochrome black-T is the metal ion indicator generally used in the determination of hardness. When Eriochrome black –T, a complex organic compound is added to hard water having buffer solution pH 10, it gives wine red colored unstable complex [MIn]- with Ca2+ and Mg2+ ions of the water sample. M2+ + [HIn]2aq, blue [MIn]- + (wine red unstable complex) H+ wherw M2+=Ca2+, Mg2+ When this wine red color solution is titrated against N/100 EDTA solution, the color of solution changes from wine red to original blue color at the end point due to the libration of indicator [HIn]2-. The metal EDTA complex is more stable than Metal indicator complex. Both [MIn]- & [MEDTA]2- complex are stable only in alkaline medium, in highly alkaline medium Mg2+ gets precipitate as Mg(OH)2 and therefore complex reaction is studied around pH 10 only. This pH range can be maintained by adding ammonical buffer (NH4Cl+NH4OH). [MIn]- + [H2EDTA]2(wine red complex) [MEDTA]2- + [HIn]2- + H+ (colourless) (blue) In this experiment water sample is boiled, cooled and filtered to remove temporary hardness causing salts: Ca(HCO3) 2 CaCO3 + H2O + CO2 The permanent hardness is determined by titration of known volume of boiled hard water sample with standardized Na2H2EDTA solution. Procedure: Step I Titration with Standard Hard water 1. Pipette out 20 ml of Standard hard water in a conical flask. Add 2 ml of buffer solution and 2 drops of Eriochrome Black –T indicator. The color of the solution becomes wine red. 2. Titrate the solution against N/100 EDTA solution with continuous shaking till the color changes from wine red to blue. 3. Note down the volume of EDTA from the burette. 4. Repeat the titration till three concordant readings are obtained. Step II Titration with (Unknown) hard water Titrate the unknown hard water sample by the procedure (step I) Step III Titration with Boiled Hard Water Titrate the Boiled hard water sample by the procedure (step I) Observation: Wine red color change in to Blue color at the end point. Observation Table: With Standard Hard water S.No. Volume of the Standard hard water taken in titration flask Burette readings Initial Reading Final Reading Volume of the EDTA solution used (V1 mL) 1 2 3 With Unknown hard water sample No. Volume of the (Unknown) hard water taken in titration flask Burette readings Initial Reading Final Reading Volume of the EDTA solution used (V2 mL) 1 2 3 With Boiled hard Water S.No. Volume of the boiled hard water taken in titration flask Burette readings Initial Reading Final Reading Volume of the EDTA solution used (V3 mL) 1 2 3 Calculation: (i) 1 ml of standard hard solution = 1 mg of CaCO3 V1 ml of EDTA=20 ml of Standard hard water 1 ml of EDTA= 20/V1mg of CaCO3 (ii)20 ml of Hard water solution = V2 mL of EDTA =V2 x20/V1 mg of CaCO3 1 ml of HW solution = V2/V1 mg of CaCO3 1000ml of HW solution=(V2/V1 ) X1000 mg of CaCO3 Total Hardness = =(V2/V1 ) X1000 ppm (i) 20 ml of Boiled water solution = V3 mL of EDTA =V3 x20/V1 mg of CaCO3 1 ml of BHW solution = V3/V1 mg of CaCO3 1000ml of BHW solution=(V3/V1 ) X1000 mg of CaCO3 Permanent Hardness = =(V3/V1 ) X1000 ppm Temporary Hardness= Total Hardness- Permanent Hardness Result (i) Total Hardness…………..ppm (ii)Permanent Hardness…………..ppm (iii)Temporary Hardness……………..ppm Precautions: (i) (ii) (iii) (iv) The apparatus should be cleaned properly with distilled water. Reaction content should be continuously shaken. The pH should be maintained during titration. The end point should be observed correctly.