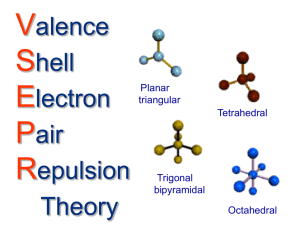
The Valence Shell Electron Pair Repulsion Theory Typical Formula Total number of electron groups around the central atom Number of Lone Pairs Number of Bonds Electron Geometry Molecular Shape AB2 2 0 2 linear linear 3 trigonal planar trigonal planar 2 trigonal planar v-shaped (bent) AB3 AB2 3 3 0 1 Bond Angles Hybrid Orbitals on Central Atom 180 sp 120 sp2 120 sp2 B 109.5 sp3 B <109.5 sp3 <109.5 sp3 Structure B A B B B A B B B .. A B AB4 AB3 4 4 0 1 4 3 tetrahedral tetrahedral A tetrahedral B ..A trigonal pyramidal AB2 4 2 2 tetrahedral v-shaped (bent) AB 4 3 1 tetrahedral linear 5 trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal see-saw trigonal bipyramidal t-shaped trigonal bipyramidal linear octahedral octahedral B B B B .. A .. ....A .. B B B AB5 AB4 AB3 AB2 AB6 AB5 AB4 5 5 5 5 6 6 6 0 1 2 3 0 1 2 4 3 2 6 5 4 octahedral octahedral B A B B .. A B B B B B B B . ... A not applicabl e 120 and 90 120 and <90 sp3d <90 sp3d 180 sp3d 90 sp3d2 <90 sp3d2 90 sp3d2 sp3 sp3d B B square pyramidal square planar .. A... . B B B B B B B B A B B ..A ..A .. B B B B B B Hints: Always start by drawing the Lewis structure and then focusing on the central atom. By "pair" we really mean group of electrons. So a double or triple bond (even though it really represents 4 or 6 electrons) counts as one group or "pair." There is a large amount of memorization associated with this concept. But, you can think logically about the shape. You should try to visualize the arrangement of total groups of electron pairs around the central atom. This will help you determine the electron geometry. Then, imagine that any lone pairs are invisible. The structure that remains is the shape of the molecule. You do need to memorize the bond angles associated with each shape. Lone pairs or non-bonded pairs of electrons are not localized between two atoms. They are not being pulled in by two nuclei, so they occupy a greater volume than bonded pairs of electrons, which are being pulled in by two nuclei. For this reason, when the central atom has lone pairs as well as bonded pairs, the bond angles are less that what they would be if the central atom had only bonded pairs of electrons. In other words, lone pairs occupy a greater volume, push the bonded pairs of electrons away, and decrease the bond angles between the bonded pairs of electrons. Only bond angles of less than 120 are affected by lone pairs. For the reasons outlined above, lone pairs will occupy equatorial positions (rather than axial positions) in trigonal bipyramidal molecules and will take positions opposite each other in octahedral molecules. When drawing a two dimensional picture of a three dimensional shape: indicates a lone or non-bonded pair of electrons. indicates a bond headed backwards into the plane of the paper or board. indicates a bond coming forward out of the plane of the paper or board. Hybridization is used to explain how the s, p, and d atomic orbitals of the central atom can be used to make molecules with bond angles other than 90. The orbitals of the central atom are said to mix together to form an equal number of hybrid orbitals that have the shape given below and the arrangement needed to form the proper bond angles. The following is the minimum that must be memorized from the other side. After knowing this, you will probably be able to "reason through" the rest. total groups electron geometry bond angles hybridization 2 linear 180 sp 3 trigonal planar 120 sp2 4 tetrahedral 109.5 sp3 5 trigonal bipyramid 6 octahedral 90 and 120 90 sp3d sp3d2