UNIT 6B Practice Test – Molecular Shapes, Molecular Polarity
advertisement

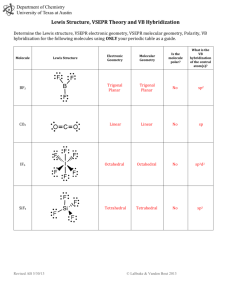
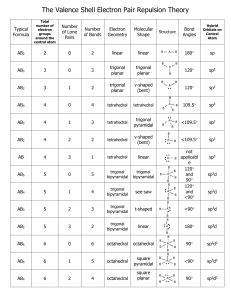
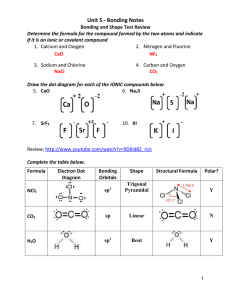
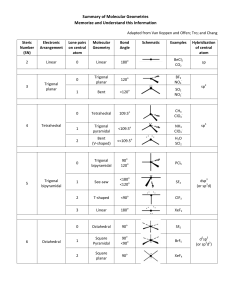
UNIT 6B Practice Test – Molecular Shapes, Molecular Polarity, Bonding Theory Total Hybridization Bonding NonDomains Domains Bonding eDomains 2 sp 2 0 Electron Geometry Molecular Geometry Linear Linear (180°)* 3 sp2 3 0 Trigonal Planar Trigonal Planar* 3 sp2 2 1 Trigonal Planar Bent (120°) 4 sp3 4 0 Tetrahedral Tetrahedral* 4 sp3 3 1 Tetrahedral Trigonal Pyramidal 4 sp3 2 2 Tetrahedral Bent (109°) 5 sp3d 5 0 Trigonal Bipyramidal Trigonal Bipyramidal* 5 sp3d 4 1 Trigonal Bipyramidal See-Saw 5 sp3d 3 2 Trigonal Bipyramidal T-Shape 5 sp3d 2 3 Trigonal Bipyramidal Linear 6 sp3d2 6 0 Octahedral Octahedral* 6 sp3d2 5 1 Octahedral Square Pyramidal 6 sp3d2 4 2 Octahedral Square Planar* Polarity of a Molecule: Must contain a polar bond/dipole (difference in electronegativity ≥ .5) Vector sum of all polar bonds must not be 0 (Molecule cannot have symmetrical dipoles) In chart above, molecular geometries with (*) can be symmetrical Valence Bond Theory: A chemical bonding theory that explains the bonding between two atoms is caused by the overlap of half-filled atomic orbitals. The two atoms share each other's unpaired electron to form a filled orbital to form a hybrid orbital and bond together. Pi π Bonds: overlap of the orbitals occurs above and below a line of the two nuclei of the atoms (present in double and triple bonds) Sigma σ Bonds: overlap of the orbitals occurs on the line between the two nuclei of the atoms (single bonds) http://www.goiit.com/upload/2009/8/27/82a63160007f42bf77f77fa2068f9728_78928.png