1 Division for Metabolic Disorders and Children`s Research Center
advertisement

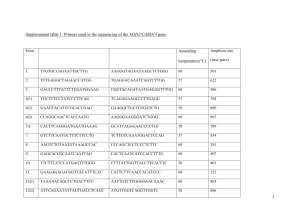
Characterization of functional domains of the cblD (MMADHC) gene product Supplementary Material Jehona Jusufi1, Terttu Suormala1, Patricie Burda1, Brian Fowler1, D. Sean Froese1*, Matthias R. Baumgartner1,2,3* 1 Division for Metabolic Disorders and Children’s Research Center, University Children’s Hospital, Zurich, Switzerland 2 radiz – Rare Disease Initiative Zurich, Clinical Research Priority Program for Rare Diseases, University of Zurich, Switzerland 3 Zurich Center for Integrative Human Physiology, University of Zurich, Switzerland *To whom correspondence may be addressed: M.R.B. Division for Metabolic Disorders and Children’s Research Center, University Children’s Hospital, Steinweisstrasse 75, 8032 Zurich, Switzerland Tel: +41 (0)44 266 7722 Fax: +41 (0)44 266 7167 Email: matthias.baumgartner@kispi.uzh.ch D.S.F. Division for Metabolic Disorders and Children’s Research Center, University Children’s Hospital, Steinweisstrasse 75, 8032 Zurich, Switzerland Tel: +41 (0)44 266 7305 Email : sean.froese@kispi.uzh.ch Supplementary Methods Transformed cblD-MMA/HC fibroblasts, grown in routine culture medium containing Dulbecco’s Modified Eagle’s Medium (DMEM)(DMEM+GlutaMax™ ; Gibco), supplemented with 10% (v/v) foetal calf serum (FCS; Gibco) and antibiotics (antibiotic-antimycotic mixture: penicillin 10'000 IU/ml, streptomycin 10'000 µg/ml, amphotericin B 25 µg/ml ) were used for transfections throughout the study. pTracerMMADHC-wt and mutant constructs were transiently transfected into these cells by electroporation. 25 µg vector DNA in a final volume of 30 µl (volume was compensated with sterile deionized water when necessary) were pipetted into a 0.4 cm gene-pulser cuvette (BioRad) followed by 300 µl cell suspension containing cells from one confluent T75cm2 culture flask in the above culture medium supplemented with 1.25% dimethylsulfoxide (Sigma) (DMSO medium), incubated at room temperature for 1 min and electroporated at 260V and 100 Ω with capacitance of 975 µF in a Gene Pulser II ( BioRad) electroporator. Cells were divided between two T25cm2 culture flasks and grown in DMSO medium. After 24 hours medium was removed, cell layers were rinsed once with 5 ml DMEM without any supplements to remove traces of FCS, and each flask was covered with aluminium foil to protect the cultures from light during the following incubation. To prepare incubation medium [57Co]-B12 Tracer Stock in 0.9% benzylalcohol (57Co-cyanocobalamin; 10.5 µCi and 25 pg/ml; ICN Pharmaceuticals Inc) was added to human serum (1 µl [57Co]-B12 Tracer Stock/ml serum) in a tube covered with aluminium foil and preincubated for 30 min at 37°C to bind the labelled cobalamin to human transcobalamin. This serum was then added to DMEM supplemented with antibiotics (1 ml serum/10 ml final medium), filtersterilized and 2 ml were added in each culture flask. Flasks were placed in cell incubator and cobalamin coenzyme synthesis was estimated after 3 days incubation. Under illumination by photo-laboratory grade red light only cells were harvested using trypsin, pelleted by centrifugation for 10 min at 1200 rpm and resuspended in 5 ml phosphate buffered saline (PBS). For estimation of total uptake of 57Cocyanocobalamin (expressed as pg/mg protein) 1 ml of cell suspensions was added into a 1.5 ml microcentrifuge tube, pelleted by centrifugation, radioactivity in pellet was quantitated in gamma counter, and protein concentration estimated by the Lowry method. For extraction of cobalamin derivatives the remaining 4 ml cell suspensions were centrifuged and pellets were spiked with 20 µl of a solution containing unlabelled AdoCbl, MeCbl, CNCbl and OHCbl (each 1 mg/ml deionized water; Sigma). Cells were lysed by 3 cycles of freezing on dry-ice (5 min) and thawing in a 37°C water bath. Extraction of cobalamin derivatives was performed by addition of 1 ml absolute ethanol to the lysed cells with incubation at 80°C in a heating block for 5-10min, centrifugation for 5 min at 1400 rpm, transfer of the supernatant to a fresh tube, addition of a further 1 ml absolute ethanol and a repeat of the procedure. The ethanol was then evaporated at 80°C in a heating block by flushing with nitrogen gas. The extracted cobalamins were dissolved by letting them stand in 80 µl buffer A (0.05 M phosphoric acid/ammonium pH 3.0) at room temperature for at least 15 min with 2-3 times Vortexmixing. Samples were transfered into microcentrifuge tubes and centrifuged for 5 min at 14000 rpm in a benchtop Eppendorf microcentrifuge to remove undissolved materials. The supernatant was transferred into a new microcentrifuge tube and different cobalamin derivatives were separated by HPLC (Jasco) from 50 µl of sample using a Lichrosorb RP-C8 column (Supelco) and gradient elution at 25°C. The separation technique was modified from the original method (Jacobsen et al. Anal Biochem 120: 394-403, 1982) to separate AdoCbl (retention time 25-27 min) from an unknown radioactive compound eluting at 23-25 min. This was achieved by the following gradient elution, with buffer A (0.05 M phosphoric acid/ammonium pH 3.0) and buffer B (buffer A supplemented with 30% v/v acetonitrile): between 0 and 15 min after injection linear change from 100% A to 46% A + 54 % B which was retained for 10 min; from 25 min to 26 min change to 100% B which was retained for 10 min; between 36 min and 37 min change from 100% B to 100% A which was retained for at least 10 min before the next injection. Fraction collection was started 15 min after injection and 48 fractions were collecetd, each for 22 seconds, and counted. The retention times of hydroxocobalamin (OHCbl; 17-19 min), CNCbl (18-20 min), AdoCbl and MeCbl (30-31 min) were estimated by detecting unlabelled compounds added to the cell pellet before extraction at 254 nm. The sum of counts per minute (cpm) in fractions belonging to each cobalamin peak was calculated and the relative concentration of each cobalamin compound was expressed as % of total cpm in all peaks (% of total cobalamins). Each mutant constructs was transfected in at least 3 independent experiments. Transfections with an empty pTracer vector, with MMADHC wildtype construct and with a construct containing one of the missense mutant alleles detected in patients with cblD-HC defect (p.D246G, p.Y249C and p.L259P) were included in each experiment. Supplementary Results – Classification of Mutations Classification of mutations was performed as follows. First, we determined the percentage of methylcobalamin (MeCbl) and adenosylcobalamin (AdoCbl) to total cobalamins for each experimental mutation and control (see above). The percentage MeCbl and AdoCbl for each experimental mutation was then compared against those from each control: empty vector, wild-type, and cblD-HC, using a twosided T-test (see Table 1). Experimental mutations that had a non-significant difference for both AdoCbl and MeCbl compared to either vector-only, wild-type or cblD-HC were placed into the category of that control (e.g. p.D226A showed non-significant differences of MeCbl and AdoCbl levels against cblD-HC (p.L259P), and was therefore categorized as cblD-HC). If the experimental mutation had a significant difference for MeCbl and/or AdoCbl levels against all 3 controls, they were then placed into a newly created category (e.g. p.W189A was significantly different from all 3 controls for both MeCbl and AdoCbl). Experimental mutations that were significantly different from the controls but were similar to each other were placed into the same category (e.g. p.P154A, p.E167A and p.K263A all had wild-type like AdoCbl [non-significant difference] but significantly different MeCbl levels from all 3 controls). Finally, the three new categories were given names stemming from the cellular or biochemical phenotypes expected due to the relative levels of MeCbl and AdoCbl, namely: “mild-MMA/HC” for the mutation that had lower MeCbl and AdoCbl than wild-type vector; “partial cblD-HC” for the mutations that had lower MeCbl but higher AdoCbl than wild-type (a cblD-HC like pattern, but with MeCbl levels slightly but significantly different from cblD-HC mutations); and “mild cblD-HC” for those mutations with only slightly lower MeCbl but much higher AdoCbl levels compared to wild-type (a cblD-HC like pattern, but with MeCbl severely and significantly different from cblD-HC mutations). The authors note that while the grouping of these latter three categories was based on statistical comparisons, the naming is arbitrary. Supplementary Table 1. Primer sequences of generated missense mutations and C-terminal truncations using site-directed mutagenesis. The sequence of the forward (normal) and reverse complementary (italic) primers are shown from 5’ to 3’. Base pairs that were changed are marked red. Codons for mutations are underlined. Nucleotide change Protein c.460C>G p.P154A c.493_494delTTinsGC p.F165A c.500A>C p.E167A c.556_557delATinsGC p.M186A c.565_566delTGinsGC p.W189A c.589_590delAGinsGC p.R197A c.610_611delTTinsGC p.F204A c.634_635delTGinsGC p.C212A c.677A>C p.D226A c.709_710delTAinsGC p.Y237A c.787_788deAAlinsGC p.K263A c.796_797delCGinsGC p.R266A c.808_809delTGinsGC p.W270A c.832_833delAGinsGC p.S278A c.838_839delTTinsGC p.F280A c.783C>A p.C261* c.817_819delCATinsTGA p.H273* c.826_827delGTinsTG p.V276* c.841_843delACTinsTGA p.T281* c.859_861delACinsTGA p.S287* Primer sequence (5’ to 3’) GTGTGCAATACAAACATGTGCAGAATTGCTGCGA ATCTTTTCGCAGCAATTCTGCACATGTTTGTAT AAAGATTTTGAATCACTGGCTCCAGAAGTAGCT GCCATTAGCTACTTCTGGAGCCAGTGATTCAAA TTTGAATCACTGTTTCCAGCAGTAGCTAATGGC TAGTTTGCCATTAGCTACTGCTGGAAACAGTGA CAAAAAACTAAGAATGATGCGACTGTTTGGAGTG CTTCTTCACTCCAAACAGTCGCATCATTCTTAGT AAGAATGATATGACTGTTGCGAGTGAAGAAGTA AATTTCTACTTCTTCACTCGCAACAGTCATATC GAAGAAGTAGAAATTGAAGCAGAAGTGCTCTTA CTTTTCTAAGAGCACTTCTGCTTCAATTTCTAC GAAGTGCTCTTAGAAAAGGCCATCAATGGTGCT TTCCTTAGCACCATTGATGGCCTTTTCTAAGAG AATGGTGCTAAGGAAATTGCCTATGCTCTTCGA CTCAGCTCGAAGAGCATAGGCAATTTCCTTAGC TATTGGGCTGACTTTATTGCCCCATCATCTGGT TGCCAAACCAGATGATGGGGCAATAAAGTCAG TTGGCATTTTTTGGACCAGCTACAAACAACACT AAAAAGAGTGTTGTTTGTAGCTGGTCCAAAAAA GATGACCTTGGATGCTGTGCAGTGATTCGTCAT GAGACTATGACGAATCACTGCACAGCATCCAAG GGATGCTGTAAAGTGATTGCTCATAGTCTCTGG GGTACCCCAGAGACTATGAGCAATCACTTTACA GTGATTCGTCATAGTCTCGCGGGTACCCATGTA TACAACTACATGGGTACCCGCGAGACTATGACG ACCCATGTAGTTGTAGGGGCTATCTTCACTAAT TGTTGCATTAGTGAAGATAGCCCCTACAACTAC GTAGTTGTAGGGAGTATCGCCACTAATGCAACA GTCTGGTGTTGCATTAGTGGCGATACTCCCTAC TCTGTTGATGACCTTGGATGATGTAAAGTGATT ATGACGAATCACTTTACATCATCCAAGGTCATC ATAGTCTCTGGGGTACCTGAGTAGTTGTAGGG GATACTCCCTACAACTACTCAGGTACCCCAGAG TGGGGTACCCATGTAGTTTGAGGGAGTATCTTC ATTAGTGAAGATACTCCCTCAAACTACATGGGT TTGTAGGGAGTATCTTCTGAAATGCAACACCA GCTGTCTGGTGTTGCATTTCAGAAGATACTCCC ACTAATGCAACACCAGACTGACATATTATGAAG TAATTTCTTCATAATATGTCAGTCTGGTGTTGC Location Exon 5 Exon 5/6 Exon 5/6 Exon 6 Exon 6 Exon 6 Exon 6/7 Exon 7 Exon 7 Exon 7/8 Exon 8 Exon 8 Exon 8 Exon 8 Exon 8 Exon 8 Exon 8 Exon 8 Exon 8 Exon 8