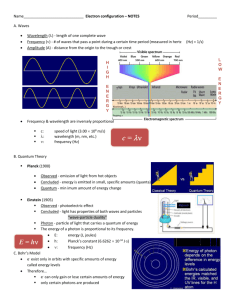
Unit 2: Electrons in Atoms Worksheet
advertisement

Name _______________________________ Spectral Lines Directions: Date ________________ Mrs. Hutchinson Bright-Line Spectra differ for each element and are used to identify which elements are involved in creating a mixture. 1. Given the bright-line spectra of the three elements and the spectrum of a mixture formed from at least two of these elements: Identify which elements are present in this mixture. 2. The diagram below represents the bright-line spectra of four elements and a bright-line spectrum produced by a mixture of two of these elements. Which two elements are in this mixture? 3. The Bright-Line Spectra for three different elements and a mixture are shown below. A. Identify all the elements in the mixture. B. Explain, in terms of both electrons and energy, how the bright-line spectrum of an element is produced. Name _______________________________ Quantum Numbers Date ________________ Mrs. Hutchinson Directions: Use your notes to help answer these questions. 1. Define what the n quantum number stands for and calculate the number of electrons that n = 11 can hold. 2. When identifying sublevels using the principle quantum number what is the general rule that you abide by to determine what your ℓ quantum number is equal to. 3. Identify the ℓ quantum number that corresponds to each sublevel. Sublevel s p d f ℓ quantum number 4. When n = 4, what sublevel(s) is/are possible? 5. The m quantum number states how many orbitals are present in each sublevel. What is the general rule in determining the number of orbitals that are present in each sublevel. 6. p = ___ ___ ___ -1 0 +1 Using the example above, draw and correctly label orbitals present in an f-subshell. 7. Identify the four quantum numbers for the following electrons ___ 1s ___ ___ ___ ___ 2s 2p ___ 3s ___ ___ ___ 3p 8. Each orbital can hold a maximum number of how many electrons? Create an equation for determining the number of electrons each orbital can hold. Name _______________________________ Orbital Filling Date ________________ Mrs. Hutchinson Directions: Draw the electron configurations for the elements in question like the example below. Be = 1s22s2 1. Nitrogen (N) 2. Cobalt (Co) 3. Potassium (K) 4. Rubidium (Rb) 5. Manganese (Mn) 6. Selenium (Se) 7. Neon (Ne) 8. Krypton (Kr) 9. Zinc (Zn) 10. Carbon (C) Name _______________________________ Ground state vs. Excited state Date ________________ Mrs. Hutchinson Directions: Determine whether the following configurations indicate an atom in a ground state or an excited state and identify the element. 1. 2 – 8 – 4 2. 1s22s22p63s23p54s1 3. 1s22s22p6 4. 2 – 8 – 17 – 2 5. 1s22s22p63s23p4 6. 1s 2s 2p 2s 2p 7. 2 – 7 – 1 8. 1s22s12p4 9. 1s 10. 2 – 8 – 18 – 8 3s Name _______________________________ Atom or ion Date ________________ Mrs. Hutchinson Directions: Identify whether the electron configuration is an atom or an ion. 1. Oxygen: 2 – 8 2. Phosphorus: 1s22s22p63s23p3 3. Sodium: 1s 2s 2p 3s 2p 3s 4. Magnesium: 2 – 8 5. Potassium: 1s22s22p63s23p6 6. Sulfur: 1s 2s 7. Germanium: 2 – 8 – 18 – 4 8. Boron: 1s22s22p1 9. Beryllium: 1s 10. Iron: 2 – 8 – 14 – 2 2s 3p