Accuracy&PrecisionSept
advertisement

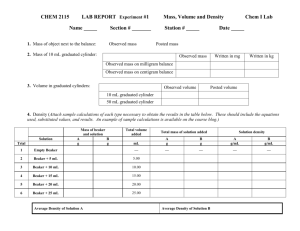
ACCURACY AND PRECISION OF VARIOUS DENSITY MEASUREMENTS Introduction: Mass and volume are extensive physical properties which depend on the quantity of substance. Density, which is the ratio of mass to volume (D = m/V), is an intensive physical property. The density of a substance, regardless of sample size, is a constant at a given temperature and is characteristic of the substance. Therefore, density can be used to aid in the identification of the substance. In this lab the mass and volume of water will be measured using different types of measuring equipment with varying degrees of accuracy, and the density of the water will be calculated. It is important for students to pay close attention to properly recording these measurements so that they correctly reflect the degree of accuracy of the measurement, and correctly rounding the calculated values so that the proper degree of accuracy is reflected in the result. Purpose: To make and record mass and volume measurements properly, to calculate density values and round appropriately paying close attention to the accuracy and precision of the measuring equipment used. Equipment/Materials: The van will provide all materials except for 250 mL beakers which the teacher should have available. Analytical balance Toploader balance 30 mL beaker 5 mL or 10 mL pipet safety bulbs for pipetting Safety: 10 mL graduated cylinder 1 mL micropipet plastic tips for micropipets distilled water thermometers Always wear goggles in the lab. Procedure: Beaker and toploader balance 1. Place about 150 mL of distilled water in a 250 mL beaker. Measure the temperature of the water and record it in the Data Table. 2. Place a clean, dry 30 mL beaker on the toploader balance. Tare. (NOTE: This subtracts the mass of the beaker from all subsequent measurements.) 3. Using the 30 mL beaker as the measuring instrument for volume, add 1 mL of water from the 250 mL beaker to the 30 mL beaker. (NOTE: There are no graduation marks on the beaker for 1 mL so this will be an estimated measurement.) Record the mass of the water in the Data Table. (NOTE: It is extremely important that you record your measurement so that it reflects the correct degree of accuracy for the measurement throughout this lab activity.) 4. Add another 1 mL (estimated) of water to the beaker. The total volume is now 2 mL. Record the mass in the Data Table. 5. Add another 1 mL of water to the beaker. The total volume is now 3 mL. Record the mass in the Data Table. Graduated Cylinder and toploader balance 1. Place a clean, dry 30 mL beaker on the toploader balance. Tare. (NOTE: In this procedure, the beaker will be used to hold the sample but NOT to measure it.) 2. Using the 10 mL graduated cylinder as the measuring instrument for volume, add 1.0 mL of water from the 250 mL beaker to the 30 mL beaker. Record the mass of the water in the Data Table. 3. Add another 1.0 mL of water to the beaker using the graduated cylinder. Total volume is now 2.0 mL. Record the mass. 4. Add another 1.0 mL of water to the beaker using the graduated cylinder. Total volume is now 3.0 mL. Record the mass. Pipet and toploader balance 1. Place a clean, dry 30 mL beaker on the toploader balance. Tare. (NOTE: In this procedure, the beaker will be used to hold the sample but NOT to measure it.) 2. Using a 5 mL or 10 mL pipet as the measuring instrument for volume, add 1.00 mL of water from the 250 mL beaker to the 30 mL beaker. Record the mass of the water in the Data Table. 3. Add another 1.00 mL of water to the beaker using the pipet. Total volume is now 2.00 mL. Record the mass. 4. Add another 1.00 mL of water to the beaker using the pipet. Total volume is now 3.00 mL. Record the mass. Micropipet and toploader balance 1. Place a clean, dry 30 mL beaker on the toploader balance. Tare. 2. Set the micropipet to deliver 1.000 mL (1000 L) and use to measure 1.000 mL of water from the 250 mL beaker to the 30 mL beaker. Record the mass of the water in the Data Table. 3. Add another 1.000 mL of water to the beaker. Total volume is now 2.000 mL. Record the mass. 4. Add another 1.000 mL of water to the beaker. Total volume is now 3.000 mL. Record the mass. Analytical balance Repeat each of the above sets of procedures using the analytical balance in place of the toploader balance. Calculations 1. Once all measurements have been performed, calculate each density result using the formula D = m/V. Be sure to round your result so that the answer has the correct number of significant digits. 2. After calculating each of the three (3) density values for one set of measuring devices, calculate the average density for that set of devices by adding the 3 density values together and dividing by 3. 3. Research to find out the accepted value for the density of water at the temperature of your sample. Calculate the % error for each density value using the following formula: % error = accepted value – your calculated average accepted value 4. Calculate the precision (standard deviation) of each set of data. Standard deviation = (X – Xave)2 (n-1) where: X = density for each individual trial Xave = average density n = number of trials Record these results in Data Table 3. ACCURACY AND PRECISION OF VARIOUS DENSITY MEASUREMENTS Data Table Name(s): Date: Period/Lab Group: o Data: Temperature of water sample used: C Accepted value for density of water at this temperature. g/mL Data Table 1 (masses measured using the toploader balance): Volume Measuring Vessel Beaker Volume of Water Mass of Water Calculated Density D = m/V Average Density //////////////////// //////////////////// % error //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// Beaker Beaker Graduated Cylinder Graduated Cylinder Graduated Cylinder Pipet Pipet Pipet Micropipet Micropipet Micropipet Data Table 2 (masses measured using the analytical balance): Volume Measuring Vessel Volume of Water Mass of Water Calculated Density D = m/V Beaker Average Density //////////////////// //////////////////// % error //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// //////////////////// Beaker Beaker Graduated Cylinder Graduated Cylinder Graduated Cylinder Pipet Pipet Pipet Micropipet Micropipet Micropipet Data Table 3 (results of Standard Deviation calculations): Precision for: Toploader Balance Beaker Analytical Balance Graduated Cylinder Pipet Micropipet Questions: 1. Which method of determining density was most accurate? Explain. 2. Which method of determining density was most precise? Explain. 3. Did the mass shown on the analytical balance tend to decrease with time? If so, why?