Ch. 6 Notes
advertisement
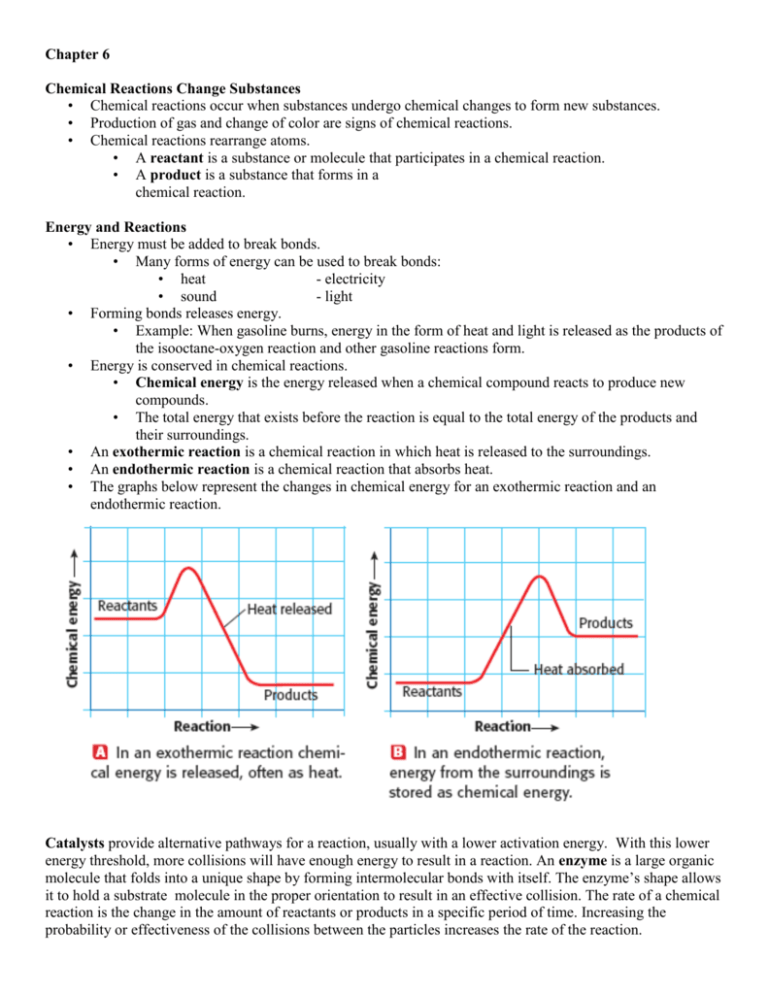
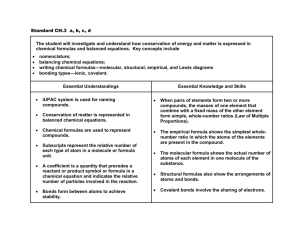
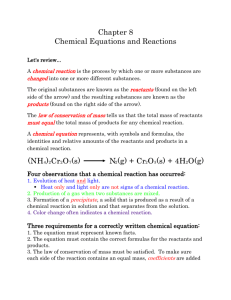
Chapter 6 Chemical Reactions Change Substances • Chemical reactions occur when substances undergo chemical changes to form new substances. • Production of gas and change of color are signs of chemical reactions. • Chemical reactions rearrange atoms. • A reactant is a substance or molecule that participates in a chemical reaction. • A product is a substance that forms in a chemical reaction. Energy and Reactions • Energy must be added to break bonds. • Many forms of energy can be used to break bonds: • heat - electricity • sound - light • Forming bonds releases energy. • Example: When gasoline burns, energy in the form of heat and light is released as the products of the isooctane-oxygen reaction and other gasoline reactions form. • Energy is conserved in chemical reactions. • Chemical energy is the energy released when a chemical compound reacts to produce new compounds. • The total energy that exists before the reaction is equal to the total energy of the products and their surroundings. • An exothermic reaction is a chemical reaction in which heat is released to the surroundings. • An endothermic reaction is a chemical reaction that absorbs heat. • The graphs below represent the changes in chemical energy for an exothermic reaction and an endothermic reaction. Catalysts provide alternative pathways for a reaction, usually with a lower activation energy. With this lower energy threshold, more collisions will have enough energy to result in a reaction. An enzyme is a large organic molecule that folds into a unique shape by forming intermolecular bonds with itself. The enzyme’s shape allows it to hold a substrate molecule in the proper orientation to result in an effective collision. The rate of a chemical reaction is the change in the amount of reactants or products in a specific period of time. Increasing the probability or effectiveness of the collisions between the particles increases the rate of the reaction. Chapter 6 Section 3 Balancing Chemical Equations Describing Reactions • One way to record the products and reactants of a reaction is to write a word equation. • Example: methane + oxygen → carbon dioxide + water • A chemical equation is a representation of a chemical reaction that uses symbols to show the relationship between the reactants and the products. • In a chemical equation, such as the one above, the reactants, which are on the left-hand side of the arrow, form the products, which are on the right-hand side. When the number of atoms of reactants matches the number of atoms of products, then the chemical equation is said to be balanced. • Balancing equations follows the law of conservation of mass. • • You cannot balance chemical equations by changing chemical formulas themselves, because that would change the substances involved. • To balance chemical equations, numbers called coefficients must be placed in front of the chemical formulas. Steps for Balancing Chemical Equations 1. 2. 3. 4. 5. 6. Count the number of atoms of each element on both sides of the equation. If the numbers are the same for each element in the equation, it is balanced. Write balanced beside the equation. If the number of atoms of each element is not the same on both sides, you must balance the equation by adding coefficients. Put a coefficient in front of a symbol or formula so that the number of atoms of that substance is the same on both sides of the equation. Continue until you have balanced all the atoms. DO NOT CHANGE SUBSCRIPTS AND DO NOT PUT COEFFICIENTS WITHIN A FORMULA !!!! Balance the Oxygen and hydrogen atoms last Check your work by counting the number of atoms of each element to make sure they are the same on both sides. If you are going back and forth more than 7 times, you probably made a mistake. Erase and start over. Maybe try something new, or start with a different element. Ex. Na + Cl2 NaCl H2 + O2 H2O Pb(NO3)2 + KI ---> PbI2 + KNO3 How to translate a chemical reaction from a written statement: Example: When calcium hydroxide reacts with hydrochloric acid, dissolved calcium chloride and water are formed. This reaction gives off heat. How to solve a problem like this: Step 1: Write the unbalanced equation by translating the written names into chemical formulas In this case, the formulas you need to know are those for calcium hydroxide, hydrochloric acid, calcium chloride, and water. When you translate these into their formulas, you should get the unbalanced equation: *Don’t forget that some compounds exist as diatomic molecules. For example if the chemical formula mentions hydrogen gas or nitrogen gas, those two elements are included in the 7 diatomics. Their formula would be H2 and N2, not just H or N by themselves. Look back to your chapter 4 notes if you forgot which elements are diatomics. Ca(OH)2 + HCl --> CaCl2 + H2O Step 2: Balance the equation You need to balance the equation to ensure that the chemical reaction follows the law of conservation of mass, which says that you've got to have the same number of atoms of each element on both sides of the equation. I'll assume for the purposes of this activity that you know how to balance equations. For this reaction, the equation, when balanced, looks like this: Ca(OH)2 + 2HCl --> CaCl2 + 2H2O Step 3: Figure out the states of each of the chemicals in the equation "States" refers to the form in which you can find a chemical. The states you need to worry about are solid, liquid, gas, and aqueous. Solid, liquid, and gas are probably familiar to you, and "aqueous" is just a fancy word for "dissolved in water". The symbol for a solid is (s), liquid is (l), gas is (g), and aqueous is (aq). You need to make sure you write these in the parentheses, and that you write them right after the formulas in the same place and size that you put the subscripts in the formulas. Now, the big question is this: How can you tell if something is a solid, liquid, gas, or aqueous? Here are some guidelines that might help you: *The equation might tell you. For example, if something is "dissolved in water", you know it's aqueous. If something is a "powder", this indicates that it's a solid. "Vapors" are gases. The term precipitate is a fancy term for a solid that is formed from a reaction. *Some chemicals are so common that you should be able to figure it out. You should know that carbon dioxide is a gas because you learned that you breathe it out of your lungs after you breathe in oxygen. Likewise, you should have a pretty good idea that water is generally a liquid, except at very low temperatures (when it is solid ice) or very high temperatures (as gaseous steam). *If you're not explicitly told otherwise, assume that ionic compounds are solids. That's because ionic compounds have very high melting and boiling points, so they usually are. If they're dissolved in water or in some other form, the equation should tell you. *If you're not explicitly told otherwise, assume that covalent compounds are liquids. This is actually not that great an assumption because there are a lot of exceptions to this rule, but it's better than nothing. Generally, covalent compounds have fairly low melting and boiling points, and many organic compounds are liquids at room temperature. Still, this is just a vague rule of thumb, and won't always work. *All metallic elements but mercury are solids. Mercury is a liquid. *All nonmetallic elements are solids, except for the following: Bromine is a liquid; * The noble gases, chlorine, fluorine, nitrogen, oxygen, and hydrogen are gases. * all acids are aqueous (bases are also aqueous but we haven’t learned how to identify those yet) *Solutions can occur in any of the three states of matter: solid, liquid, and gaseous. The most common solutions are liquid, composed of a solid, liquid, or gas dissolved in a liquid solvent. Other solutions result from the dissolving of one gas in another, a liquid in a gas, or even one solid in another. (Solid solutions are formed while the materials—copper and zinc for brass—are heated to the liquid state.) Water is the most abundant and widely used solvent. Its solutions are usually called aqueous solutions So, let's take a look at our equation: (In case you forgot what it was, it was Ca(OH)2 + 2HCl --> CaCl2 + 2H2O Calcium hydroxide is an ionic compound, so we'll assume it's a solid. Hydrochloric acid is an acid so its aqueous . The equation tells us that calcium chloride is dissolved in water, so it's aqueous. Water is a liquid, because nothing about the statement told us that the reaction took place at anything but room temperature. Putting all this stuff together, we get the following equation: Ca(OH)2(s) + 2HCl(aq) --> CaCl2(aq) + 2H2O(l)