Chemical symbols.
advertisement

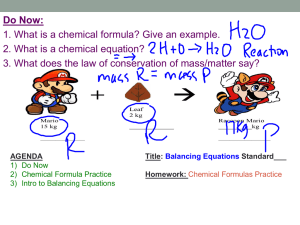
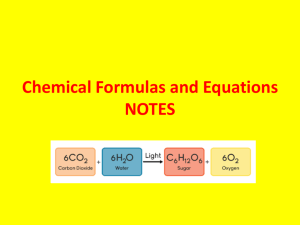
A.6 Symbols, Formulas And Equations - Chemical language used in oral and written communication has been developed to represent atoms, elements, and compounds. 1. The “letters” in this language’s alphabet are Chemical symbols. - Each element is assigned a chemical symbol. - Only the 1st letter of the symbol is capitalized; all other letters are in lowercase. For example, C is the symbol for the element carbon and Ca is the symbol for the element calcium. 2. “Words” in the language of chemistry are composed of “letters” (symbols representing elements). Each “word” is a chemical formula, which represents a different chemical compound. - In the chemical formula of a compound, a chemical symbol represents each element present. - A Subscript (a small number written below the normal line of letters) indicates how many atoms of the element. Example, ammonia NH3. The subscript 3 indicates that each ammonia molecule contains three hydrogen atoms. Ammonia also contains one nitrogen atom. The subscript 1 is understood in the absence of any subscript and is not included in chemical formulas. 3. If formulas are words in the language of chemistry, then Chemical equations can be regarded as chemical “sentences” - Chemical equations summarizes the details of chemical reaction. Chemical reactions entail the breaking and forming of chemical bonds, causing atoms to become rearranged into new substances. These new substances have different properties from the original materials. 3 H2 + N2 Hydrogen Nitrogen gas gas 2 NH3 Ammonia Reactants Product What was produced: 3 hydrogen molecules (H2), and one nitrogen molecule (N2) reacted to produce ( ) two molecules of ammonia (NH3). Reactants(LS of arrow) are the original substances in a chemical reaction. Products(RS of arrow) are the new substance(s) formed from the rearrangement of the reactant atoms. Balanced total number of each type of atom is the same for both reactants and products.