Supporting Information Ratiometric fluorescence chemosensors for
advertisement

Supporting Information
Ratiometric fluorescence chemosensors for HSO4- ion based on pyrrole-substituted
salicylimine based Zn2+ complex: nanomolar detection
Umesh Fegadeac, Jitendra Bhosalea, Hemant Sharmab, Narinder Singhb, Ratnamala Bendrea, Anil Kuwara*
a
b
School of Chemical Sciences, North Maharashtra University, Jalgaon, 425001 (MS) India.
Department of Chemistry, Indian Institute of Technology, Ropar, Rupanagar, Punjab, India
*Corresponding author. E-mail addresses: kuwaras@gmail.com, bendrers@rediffmail.com.
---------------------------------------------------------------------------------------------------------------Stoichiometry of complexations
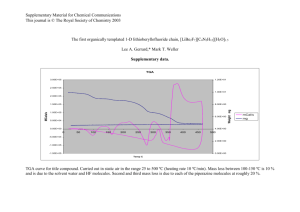
To determine the binding stoichiometry between receptor 4 and HSO4- the continuous variation
method was used. Fig. S7 shows the Job’s plot of the fluorescence intensity of free receptor 4 and the
intensity of the system with the molar fraction of the host {[H]/([H]+[G])} for a series of solutions, in
which the total concentration of host and guest was constant, with the molar fraction of host
continuously varying. The results show that the formation of a 1:1 (Host: Guest) complex. Using the
equation: [G]tot = a/2K(1-a)2[H]tot + a[H]tot/2, where [G]tot is total concentration of guest, [H]tot is the
total concentration of host, a = (I- I0)/(Ii- I0) with I being the fluorescent intensity at a particular guest
ion concentration while I0 and Ii are the intensities at zero and infinite guest concentrations, respectively.
0.00016
0.00014
0.00012
[HG]
0.00010
0.00008
0.00006
0.00004
0.00002
0.00000
0
0.2
0.4
0.6
0.8
1
[H]/[H]+[G]
Figure S1. 1:1 Stoichiometry of the host guest relationship realised from the Job’s plot for receptor 4.
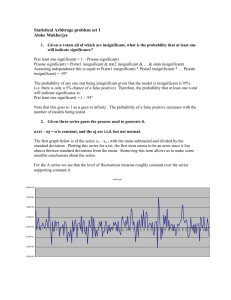
The Benesi-Hildebrand Plot
Using the Benesi-Hildebrand Plot (Eq.1) methodologies. We made the calculation of association
constant K.
1/F-F0 = 1/(F∞ - F0)K[G] + 1/(F∞-F0)
(Eq.1)
1/F-Fo
200000
180000
160000
140000
120000
100000
80000
60000
40000
20000
0
0.00E+00
y = -5.189x + 202353
R² = 0.9802
2.00E+04
4.00E+04
1/G
Figure S2. Benesi-Hildebrand Plot receptor 4 (adjusted equation: 1/F-F0 = -5.189+20235/[G], R=0.980) at
the K value 38997 M-1.
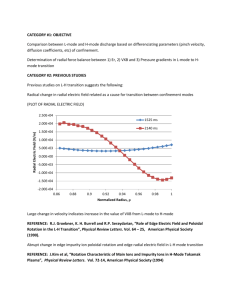
The Stern–Volmer quenching constant
The quenching can be mathematically expressed by the Stern–Volmer Eq. (2), which allows for
calculating quenching constants.
F0/F = = 1 + kq τ0 [Q] = 1 + Ksv[Q]
(2)
Where F0 and F are the fluorescence intensities in the absence and presence of the quencher, kq is the
bimolecular quenching constant, τ0 is the lifetime of the fluorescence in the absence of the quencher [Q]
is the concentration of the quencher, and Ksv is the Stern–Volmer quenching constant. In the presence of
a quencher (Lns), the fluorescence intensity is reduced from F0 to F. The ratio (F0/F) is directly
proportional to the quencher concentration [Q].
Evidently:
Ksv = kq τ0
(3)
F0/F = 1 + Ksv[Q]
(4)
According to Eq, a plot of F0/F versus [Q] shows a linear graph with an intercept of 4 and a slope of Ksv.
A typical plot of F0/F versus HSO4- concentration is shown in Fig S3.
y = 11642x + 0.8868
R² = 0.9824
3.50E+00
3.00E+00
Fo/F
2.50E+00
2.00E+00
1.50E+00
1.00E+00
5.00E-01
0.00E+00
0.00E+00
5.00E-05
1.00E-04
1.50E-04
2.00E-04
Q(M)
Figure S3. Stern–Volmer plots for titrations of receptor 4 with different concentrations of HSO4- anion.