Chapter 10: States of Matter
advertisement

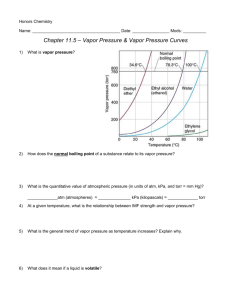
CP Chemistry Chapter 10: States of Matter Name: _____________________________ Mods: ______________ Chapter 10: States of Matter Reading Guide 10.1 – The Kinetic Molecular Theory of Matter (pgs. 311-314) 1. The kinetic-molecular theory (KMT) is based upon the idea that ________________ of matter are ________ in _____________. 2. The kinetic-molecular theory explains the constant motion of gas particles. The theory provides a model of what is called an ideal gas. Define ideal gas: Define elastic collision: 3. Summarize the five (5) assumptions that kinetic-molecular theory is based on: (2 -3 sentences each) (1) (2) (3) (4) (5) 4. What happens to the average speed of gas particles as their temperature is increased? 5. Answer the below questions from what you have read so far about KMT: (a) What would happen if you transferred a quantity of a gas from a 1-liter bottle to a 2-liter bottle? (b) Why are liquids and gases both considered fluids? (c) Why do gases have such low densities as compared to liquids and solids? 6. Explain the difference between effusion and diffusion 7. Define a real gas: 8. Critical thinking: Look at graphic 1.3 on page 314. What will eventually happen to the gas if it is compressed enough? 10.2 – Liquids (pgs. 315-318) 9. What determines the properties of liquids? 10. Define surface tension and provide an example: 11. Define capillary action and provide an example: 10.3 – Solids (pgs. 319-323) 12. Complete the matching section below. a. b. covalent network crystals metallic crystals c. covalent molecular crystals d. ionic crystals ________ 1. consists of covalently bonded molecules held together by intermolecular forces. ________ 2. consists of positive and negative ions arranged in a regular pattern. ________ 3. metal cations surrounded by a sea of delocalized valence electrons. ________ 4. each atom is covalent bonded to its nearest neighboring atoms. 10.4 – Changes of State (pgs. 319-323) 13. During phase changes, the states of matter are in equilibrium with one another; they change back and forth between the states of matter at equal speeds 14. Define the following terms: Phase: 15. A gas in contact with its liquid or solid phase is called a ___________________. 16. Review figure 4.2. Describe the rates of evaporation and condensation when they are at equilibrium: States of Matter: the Molecular Perspective 1. States of Matter – “definite” or “indefinite” volume and shape State of Matter Volume Shape 2. States of Matter – Visual representation of how the atoms/molecules are organized Solid: Liquid: very ordered arrangement particles in fixed positions does not flow particles vibrate in place very close to one another ridged crystalline structure retains its shape & volume virtually incompressible condensed phase Gas: less ordered arrangement particles are free to move readily flows particles move quickly particles are close together assumes shape of container constant volume virtually incompressible condensed phase total disorder of particles readily flows particles move extremely fast particles very far apart complete freedom of motion assumes shape of container variable volume much empty space easily compressed 3. At a given temperature and pressure, the state of matter of a substance depends on 2 quantities: (1) _________________ ______________ of particles: KE is the energy of ____________________. At higher temperatures, particles will move ___________________. As a result, KE and temperature are ____________________ proportional (2) Strength of _________________________ between particles: Forces of attraction bond particles together. Generally, the closer together the particles are to each other, the ______________________ the attraction between them How does KE and strength of attraction explain properties of solids, liquids, and gases? Solids – have very ____________ KE (particles vibrate in place) and have very ________________ forces if attraction; this keeps the partciles tightly bound together in a ridged, orderly structure Gases – have very ____________ KE (particles are fast moving) and have very ________________ forces of attraction because the particles are so far apart from one another 4. Phase Changes – there are two ways to change the state of matter of a substance: (1) Changes in temperature: When matter is heated, temperature & KE increases, causing particles to move faster and spread appart, thus ___________________ the forces of attraction (at a certain point, the KE is great enough to overcome the forces of attraction between the particles) When matter is cooled down, the oppsite occurs and the forces of attraction become ___________________ (now the KE is not great enough to overcome the attraction) (2) Changes in pressure: Applying pressure to matter will force the particles ____________________ to one another, reducing their motion and thus ___________________ the strength of attraction between the particles Typically the _____________ state of matter is most affected by to changes in pressure Change of State solid liquid liquid gas solid gas gas solid gas liquid liquid solid Process Example Phase Diagrams – In Class Notes 1) A phase diagram is a graph of pressure vs. temperature that shows the conditions at which the phases of the substance exist. It also shows how the phases change with changing temperatures and pressures. 2) Key Parts of a Phase Diagram: a) States of Matter: Solids exist at _____________ pressures and ____________ temperatures, while gases exist _____________ pressures and ____________ temperatures b) Atmospheric Pressure: This is the pressure our atmosphere exerts on us (at sea level). There are several units for pressure, but all of the following are equal each another and reflect atmospheric pressure: ____atm (atmosphere) = ______torr = _______mm Hg (millimeters of mercury) = ___________kPa (kilopascal) c) Melting & Boiling Points: Melting Point – the temperature at which a substances changes from a solid to liquid; this phase change occurs when the solid and liquid states are in equilibrium with one another Boiling Point – the temperature at which a substances changes from a liquid to gas; this phase change occurs when the liquid and gas states are in equilibrium with one another o All substances have only one NORMAL melting point and one NORMAL boiling point which occur at __________________________ pressure o If the pressure is changed to something other than atmospheric pressure, then the substance will have different melting and boiling points d) Triple Point – indicates the temperature & pressure conditions at which the solid, liquid, and gas phases of a substance _______________ at the same time in equilibrium with each other e) Critical Point - the temperature & pressure at which there is no longer distinct ________________ and ________________ phases Once a substance reaches the critical point or exceeds the critical temperature or critical pressure, it becomes a supercritical fluid Supercritical Fluid – the substance exhibits behaviors of both liquids and gases (it can behave as a gas, both effusing & diffusing, but it can also dissolve other substances like a liquid). Phase Diagram of Bromine (Br2) – In Class Example [Note that the scales are distorted to emphasize some of the graph’s features] Directions: Answer the questions below using the phase diagram of bromine. 1) Label the states of matter in the appropriate places on the phase diagram above. 2) Identify the phase changes that occur on the line next to points A, B, and C on the diagram. A: __________________________________and __________________________________ B: __________________________________and __________________________________ C: __________________________________and __________________________________ 3) Label bromine’s normal melting point (MP), normal boiling point (BP), triple point (TP), & critical point (CP) on the diagram & estimate the temperature & pressure values at each point: Temperature (ᵒC) Pressure (kPa) Temperature (ᵒC) Normal MP Triple Point Normal BP Critical Point Pressure (kPa) 4) Looking at the diagram, describe what happens to the melting point of bromine as the external pressure increases. What can you conclude about the forces of attraction between the bromine molecules as the pressure increases? What is the relationship between the melting point (or boiling point) of a substance and the strength of attractive forces between its molecules? 5) If you keep the temperature of a substance constant, but increase the pressure the more dense state of matter is always favored. If you look at the slope of the melting-point equilibrium line, a positive slope (as seen with bromine) means that with increasing pressure will favor the _________________ state as the more dense state of matter. Rarely, a substance may have a negative slope signifying that the ________________ state of matter would be denser. An example of a substance like this would be _____________________ 6) What is the boiling point of bromine when the external pressure is 75 kPa? _______________ 7) Complete the following sentences by filling in to correct phase change that occurs: a) Bromine gas at 15°C will ______________________________ when the pressure is raised from 10 kPa to 50 kPa. b) Br2 liquid at 70 kPa _______________________________ when the temperature is decreased from 20°C to –15°C. c) Bromine solid at –20°C will undergo _____________________________ is the pressure is increased from 0 kPa to 10 kPa. Phase Diagram of Water – In Class Example 1) Describe all the phase changes a sample of solid water would undergo when heated from 10°C to its critical temperature at a constant pressure of 1.00 atm. 2) Describe all the phase changes a sample of water vapor would undergo when cooled from 110°C to 5°C at 1.00 atm of pressure. 3) At what pressures will water be a vapor at 0°C? ___________________________________ 4) Within what range of pressures will water be a liquid at temperatures above its normal boiling point? Phase Diagrams – Homework WS #1 Directions: Refer to the phase diagram below when answering the questions on this worksheet: 1) At atmospheric pressure at room temperature (27ᵒC), what state of matter is this substance? 2) What is the normal melting point of this substance? _________________ 3) What is the normal boiling point of this substance? _________________ 4) What is the normal freezing point of this substance? _________________ 5) If I had a quantity of this substance at a pressure of 1.25 atm and a temperature of 300ᵒC and lowered the pressure to 0.25 atm, what phase transition(s) would occur? 6) At what temperature and pressure do the gas and liquid phases become indistinguishable from each other? What is this point called? 7) If I had a quantity of this substance at a pressure of 0.50 atm and a temperature of –100ᵒC, what phase change(s) would occur if I increased the temperature to 600ᵒC? At what temperature would this occur? 8) Under what conditions of temperature and pressure are all three states of matter in equilibrium? What is this point called? Phase Diagrams – Homework WS #2 Use the phase diagram for water below to answer the following questions. 1) Label the three states of mater on the diagram above. 2) What is standard atmospheric pressure on this graph? _______________________ [look at units] 3) What is the normal melting point of this substance? _____________________ 4) What is the normal boiling point of this substance? _____________________ 5) At atmospheric pressure and room temperature (27°C), what state of mater is this substance? 6) If the external pressure was raised to 160 kPa, what would be the new melting and boiling points of this substance? 7) What phase change occurs when a substance at 60 kPa and 0°C has its pressure lowered to 10 kPa? 8) At what temperature and pressure is the critical point? What is the critical point? Phase Diagrams – Homework WS #3 Use the phase diagram for water below to answer the following questions. Carbon dioxide is a unique substance, hence its very a-typical phase diagram. The states of matter have been labeled to help assist you in answering the questions below. 1) At atmospheric pressure and room temperature (27ᵒC), what state of matter is the substance? 2) At the point labeled -78.5ᵒC at 1 atm, what phase change is occurring? What would we call this point? 3) What is happening at the conditions of -56.6ᵒC at 5.11 atm? 4) It is very difficult to get CO2 into the liquid phase. At a temperature of –40.0ᵒC it is possible to melt solid CO2 into a liquid; what pressure would be necessary to do this? 5) Under what range of pressure conditions would CO2 be a liquid at –20.0ᵒC? Vapor Pressure Curves – In Class Example VAPOR PRESSURE GRAPHS: Use the graph shown below to answer the following questions. 1) Define vapor pressure. 2) Looking at the curves, what is the general trend for vapor pressure as temperature increases? Why does this make sense? 3) What is a volatile liquid? 4) Which substance in the graph is the most volatile? 5) What is the vapor pressure of CHCl3 at 50C? 6) What is the boiling point of H2O when the external pressure is 30 kPa? 7) What is the normal boiling point of CCl4? 8) Which substance has the weakest intermolecular forces of attraction (IMFs)? 9) Explain the relationship between IMFs and vapor pressure? Pressure, Boiling Points, and Real Life Applications 1) At sea level, atmospheric pressure is approximately 1 atm. Denver, Colorado is known as the “mile high city” since it is exactly 5280 feet above sea level. a) Do you expect the atmospheric pressure in Denver to be higher or lower than 1 atm? b) Based on your answer to part a, would you expect a pot of water in Denver to have a higher or lower boiling point than 100ᵒC? Why? c) What does this mean in terms of time it takes to cook pasta in Denver? 2) Many families use pressure cookers in order to speed up the time it takes to cook meat. a) Explain how a pressure cooker achieves this, in terms of pressures and boiling points. Vapor Pressure Curves – Homework WS #1 Use the graph shown below to answer the following questions. 1) What is the vapor pressure of the carbon disulfide at 30°C? 2) What is the boiling point of ethanol when the external pressure is 200 mmHg? 3) What is the normal boiling point of carbon disulfide? 4) What is the vapor pressure of heptane at 70°C? 5) What is the temperature if the vapor pressure of heptane is 450 mmHg? 6) Which substance on the graph has the weakest intermolecular forces of attraction? Vapor Pressure Curves – Homework WS #2 Use the graph shown below to answer the following questions. 1) What is the vapor pressure of chloroform at 50°C? _________________ 2) What is the boiling point of water when the external pressure is 30 kPa? _________________ 3) What is the normal boiling point of ethanol? _________________ 4) If ethanoic acid is boiling at 100°C, what is its external pressure? ___________________ 5) Comparing the curves, which substance (of the four given above) has the weakest IMFs? 6) At an external pressure of 60 kPa, which substance would take the longest to heat to a boil? Chapter 10: Phase Changes – Review Worksheet (Part 1) A. Matching: On the line at the left, write the term that best matches each description below: vaporization condensation melting point melting phase change sublimation equilibrium vapor pressure volatile deposition ___________________________ 1) conversion of solid directly into a gas ___________________________ 2) opposite of vaporization ___________________________ 3) temperature at which solid and liquid phases exist in equilibrium. ___________________________ 4) conversion of a substance from one of the three states of matter to another ___________________________ 5) change from a liquid to gas ___________________________ 6) pressure exerted by a constant number of gas molecules in equilibrium with their liquid phase ___________________________ 7) description of a liquid that evaporates easily, with a low boiling point and high vapor pressure ___________________________ 8) transformation of a gas directly into a solid ___________________________ 9) phase change from a solid to a liquid B. True/False: If the statement is true, write “true”. If it is false, change the underlined word or words to make the statement true. Write your answer on the line provided. ___________________________ 1) The average kinetic energy of the particles in a liquid depends upon the temperature. ___________________________ 2) Solids do not flow because the attractive forces between their particles are weaker than those in liquids or gases ___________________________ 3) Dynamic equilibrium is reached when the rate of vaporization exceeds the rate of condensation. ___________________________ 4) Condensing liquid water is the opposite of melting solid ice ___________________________ 5) All three states of matter can exist in equilibrium at the triple point C. Fill in the diagram below with the appropriate phase changes. D. Fill in the phase diagram at right with the three states of matter in the empty ovals. Then state which phase changes are be represented by letters A-F. A. _____________________________ B. _____________________________ C. _____________________________ D. _____________________________ E. _____________________________ F. _____________________________ Ch. 10: Phase Changes – Review Worksheet (Part 2) Phase Diagram for a Generic Substance: Answer the following questions in relation to the phase diagram below. 1) What section represents the solid? _______ liquid ? _______ 2) What letter represents the triple point? ________ gas? _______ critical point? ________ 3) What state is this substance in at atmospheric pressure and room temperature (27ᵒC)? 4) What phase change occurs at 1 atm and 197.5ᵒC? 5) At 240ᵒC, what phase change would occur if the pressure increases from 4 atm to 10 atm? 6) At what temperature and pressure conditions do all three phases coexist? 7) At a constant temperature, what would you do to the pressure to cause this substance freeze (liquid solid) Phase Diagram for Water: Answer the following questions in relation to the phase diagram below. 1) What is the normal freezing point of water? ____________________ 2) What is the normal boiling point of water? _____________________ 3) What is the critical temperature and pressure of water? 4) Use line segments AB, BC, or BD to answer each if the following. a) Which line segment represents melting? _________ b) Which line segment represents deposition? _________ c) Which line segment represents evaporation? _________ d) Which line segment represents sublimation? _________ 5) Identify the likely state of matter at each of the following conditions: a) 80°C and 0.081 kPa _____________________ b) 50°C and 10,000 kPa _____________________ c) -100°C and 101.3 kPa _____________________ Phase Diagram for Nitrogen (N2) Answer the following questions in relation to the phase diagram below. 1) What is the normal melting point of nitrogen? ____________________ 2) What is the normal boiling point of nitrogen? _____________________ 3) At room temperature (27ᵒC) and atmospheric pressure, what state of matter is N2? ____________ 4) In laboratories, nitrogen is purchased and delivered in its liquid state inside a pressurized canister. a) Assuming that N2 was delivered at a pressure of 5 atm, what temperature must it have been? b) When canister of liquid nitrogen is opened to standard laboratory conditions, what phase change is expected to immediately occur? c) Based on your knowledge of states of matter and melting/boiling points, would you consider nitrogen (N2) to have strong or weak intermolecular attractive forces? Explain.