Lab#2
advertisement

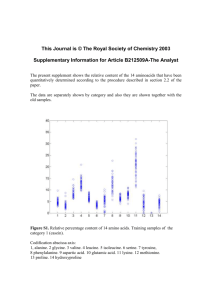
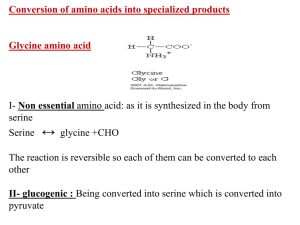
Lab # 2: Some Properties of Amino Acid and Proteins Jenna Downey Friday Lab # 16 Results: Part 1) table Biuret test Ninhydrin test Xanthoproteic test diluted egg albumin purple clear yellow 2% gelatin purple very slight pink/red Light yellow skim milk purple purple yellow with clumps at surface 0.1M glutamic acid blue clear clear 0.1M valine blue clear clear 0.025M tyrosine blue very light red/purple yellow 0.1M sucrose blue clear clear Part 2) TLC Plate Calculations: 1- Aspartic acid = 8/60 = 0.13mm 2- Glutamic acid = 10/60 =0.17mm 3 - Glycine = 16/60 = 0.27mm 4 - Valine = 52/60 = 0.87mm 5 - Phenylalanine = 36/60 = 0.6mm 6 - Unknown (#1) = 35/60 = 0.59mm Chemical Reactions: 1. An amino acid with ninhydrin imine product 2. Mono-nitration of tyrosine Questions: 1. All three proteins within the xanthoproteic test produced positive results, which included diluted egg albumin, skim milk and 2% gelatin produced yellow coloring in the protein. Gelatin is a mixture of peptides and proteins. The results of gelatin were a lighter yellow color, as it contains amino acids (proline, glycine, and hydroproline) which do not have aromatic rings, with only small trace amount of tyrosine, an amino acid containing an aromatic ring explaining for the faint yellow coloring. This conclusion came from tyrosine, being the only amino acid within this test producing a positive yellow result. tyrosine. 2. Amino acids tested in this lab are ranked from smallest to highest based upon their Rf values; Aspartic acid, Glutamic acid, Glycine, Unknown 1, Phenylalanine, and Valine. Aspartic acid and glutamic acid are polar molecules due to –OH, NH2, and C=O functional groups. Therefore they have a stronger adsorption into the silica gel. Glycine, contain same functional groups, but the amounts are decreased, therefore allowing glycine to be less polar (i.e. traveling up the TLC plate a further distance). Unknown 1 and phenylalanine show near identical polarities, due to their Rf value, concluding that they are the same amino acid. Valine, through my test showed to be lest polar. Upon research its structure shows an isopropyl side chain. When compared to phenylalanine’s benzene ring, I believe its suffice to conclude that valine should have a lower Rf value then phenylalanine although my TLC plate does not show such conclusion.