***** **** 6
advertisement

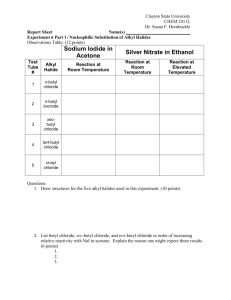
2 מתוך1 עמוד 6 ניסוי מספר Nucleophilic Substitutions. There will be 3 different substitution reactions; your lab assistant will inform you which one you will do. You will be tested on your knowledge of nucleophilic substitution reactions and reactivity of alkyl and aryl halides. You must be ready to do any of the procedures so read them very carefully. We will be using magnetic stirrers instead of boiling stones in all the procedures. 1- t-Butyl chloride HCl OH Cl + H2O Reference: Vogel’s Textbook of practical organic chemistry, 5th Ed. Procedure 5.49 Page 556. Notes: Use the calcium chloride procedure and half the amounts of reagents, also when adding sodium bicarbonate to your reaction product use an Erlenmeyer and not the separatory funnel. Only introduce the mixture in the separatory funnel once gas evolution has subsided (stopped). After you have finished and isolated your compound proceed to the reactivity of halides section below. 2- t-Pentyl chloride OH HCl Cl + H2O Reference: Introduction to Organic Laboratory Techniques, 3rd Ed. Pavia, Lampman, Kriz. Procedure 23B; Page 177. Notes: When adding sodium bicarbonate to your reaction product use an Erlenmeyer and not the separatory funnel. Only introduce the mixture in the separatory funnel once gas evolution has subsided (stopped). After you have finished and isolated your compound proceed to the reactivity of halides section below. 3- n-Butyl bromide OH + H2SO4 (aq.) NaBr Br Reference: Introduction to Organic Laboratory Techniques, A small scale approach. Pavia, Lampman, Kriz, Engel. Experiment 23A; Pages 189-191. (This is not the same procedure as last year!) Use a magnet instead of boiling stones and don’t heat the bromide with the calcium chloride. Be extremely careful when handling concentrated sulfuric acid! Only proceed to the reactivity of halides section if you have enough time (you probably 6 ניסוי מספר 2 מתוך2 עמוד won’t). If you don’t have enough time copy the results table from a lab partner and make your own conclusions about the results (include them in the report!). Reactivity of alkyl and aryl halides. You will check the reactivity of different halide substrates towards SN1 and SN2 type reactions. You will receive a 15% sodium iodide solution in acetone and a 1% silver nitrate solution in ethanol. You will test how these solutions react in a dry and clean test tube with 5 drops of: 1) n-Butyl chloride 2) n-Butyl bromide (if possible what you or a lab partner have made) 3) sec-Butyl chloride 4) t-Butyl chloride (or t-pentyl chloride) 5) Bromobenzene Add 2 ml of the sodium iodide solution to the first 5 test tubes. Note whether there is a reaction and what is the rate. Now add 2 ml of the silver nitrate solution to the other 5 test tubes. Again note if there is any change and how fast it occurs (use a stopwatch). After 5 minutes take all the tubes that did not react in the first series and heat them to 550C for 15 minutes. Note any changes. Summarize the results in a table and draw your conclusions. What are the reactions that are happening? What is the mechanism in each case? 6 ניסוי מספר