EXPERIMENT 7
EXPERIMENT 7
NUCLEOPHILIC SUBSTITUTION REACTIONS: COMPETING NUCLEOPHILES
REFERENCE: Introduction to Organic Laboratory Techniques, 3rd Ed., Pavia, Lampman and
Kriz
Experiment 24, pp. 178-183 Gas Chromatography: Quantitative Analysis, pp. 629 640
OBJECTIVES: The student should be able to:
1. Determine the percentage of each product in the reaction mixture by their refractive index, their densities, or gas chromatographs.
2. Determine which reaction mechanism the reaction is going by, from the percentage of each product produced.
INTRODUCTION:
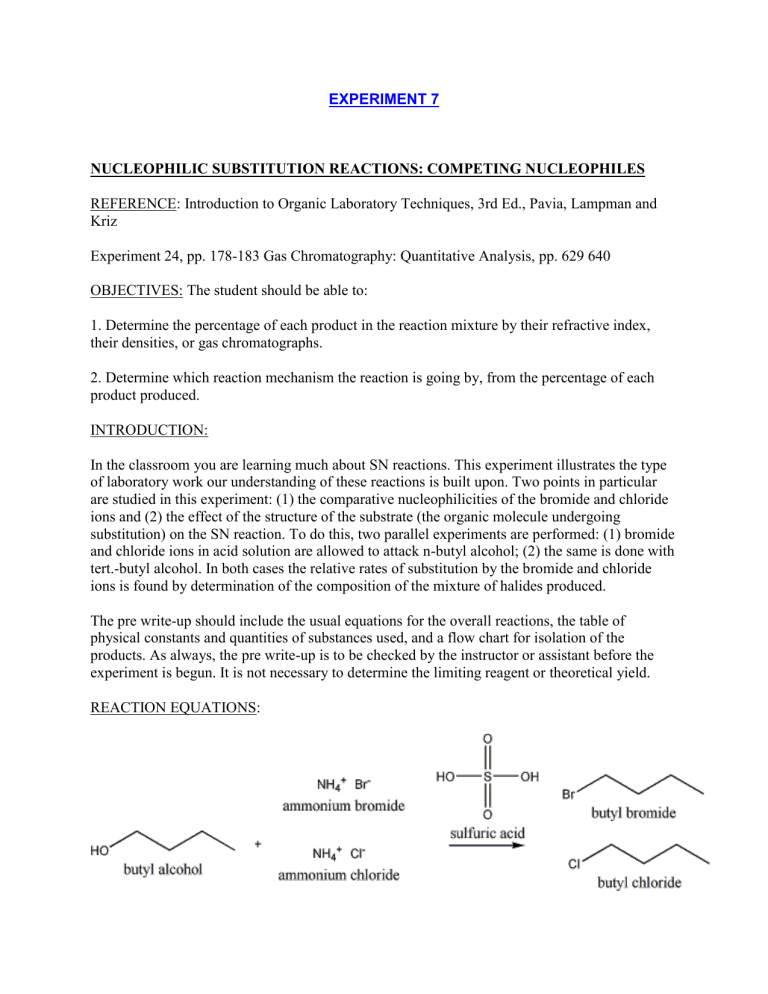
In the classroom you are learning much about SN reactions. This experiment illustrates the type of laboratory work our understanding of these reactions is built upon. Two points in particular are studied in this experiment: (1) the comparative nucleophilicities of the bromide and chloride ions and (2) the effect of the structure of the substrate (the organic molecule undergoing substitution) on the SN reaction. To do this, two parallel experiments are performed: (1) bromide and chloride ions in acid solution are allowed to attack n-butyl alcohol; (2) the same is done with tert.-butyl alcohol. In both cases the relative rates of substitution by the bromide and chloride ions is found by determination of the composition of the mixture of halides produced.
The pre write-up should include the usual equations for the overall reactions, the table of physical constants and quantities of substances used, and a flow chart for isolation of the products. As always, the pre write-up is to be checked by the instructor or assistant before the experiment is begun. It is not necessary to determine the limiting reagent or theoretical yield.
REACTION EQUATIONS:
SIDE REACTIONS:
DEHYDRATION OF THE TWO ALCOHOLS TO THE CORRESPONDING ALKENES
DIRECTIONS:
A. Solvent-Nucleophile Medium
1 Place 50 grams of ice in a 250 ml erlenmeyer flask and carefully add 38 ml of concentrated sulfuric acid. Set this mixture aside to cool. Weigh out 0.18 moles of ammonium chloride and
0.18 moles of ammonium bromide into a 250 ml beaker2. Crush any lumps of these reagents to a powder and transfer these solids to a 500 ml erlenmeyer flask using a powder funnel. Cautiously add the sulfuric acid solution to the ammonium salts, a little at a time. Swirl the flask to induce the salts to dissolve. Heat the mixture on a steam bath to achieve total solution. When solution has been achieved, allow the liquid to cool for no more than one minute3 and then pour 35 ml of the sulfuric acid-ammonium salt mixture into a 125 ml separatory funnel4 (for the t-butyl alcohol reaction) and the remainder into a 500 ml round bottom flask for reflux (for the n-butyl alcohol reaction). A small portion of the salts in the separatory funnel or the round bottom flask or both may precipitate out as the solution cools. Do not worry about these at this point; they will redissolve during the reactions.
B. SN Reaction - 1-Butanol.
Connect the 500 ml round bottom flask with the sulfuric acid-ammonium salts mixture to a condenser and add a boiling chip to the flask (use white boiling chips). Add 5 ml (0.055 moles) of 1-butanol through the condenser carefully. Start the water circulating in the condenser and then reflux gently5 for 75 minutes with a heating mantle. After reflux, cool the reaction flask in an ice-water bath before dissassembling the reflux apparatus6. The cooled mixture is transferred
to a 125 ml separatory funnel taking care to leave behind any solid precipitate. Allow the layers to separate and drain the aqueous layer. Add 10 ml of water to the organic layer(s), shake and separate6. Wash the organic layer with 10 ml of sodium hydrogen carbonate solution, separate and drain the organic layer into a 50 ml beaker containing about 0.5 grams of anhydrous sodium sulfate. When the solution is clear, decant the product solution into a 50 ml erlenmeyer flask containing a few beads of anhydrous calcium chloride7. Stopper the flask, label and turn in to the
Instructional Assistant or Instructor for Gas Chromatograph Analysis.
C. SN reaction - tert.-butyl alcohol
Measure 5 ml (0.052 moles) of t-butyl alcohol in a warm graduated cylinder and add this to the separatory funnel containing 35 ml of the warm sulfuric acid-ammonium salt solution. Stopper the separatory funnel and invert to release pressure8. After releasing pressure, turn the separatory funnel upright, swirl the funnel a couple of times and then invert it to release the mixing pressure. Repeat this until the pressures are substantially equalized; then invert the funnel and shake it vigorously with occasional venting for 2 minutes9. After shaking, allow the layers to separate for no more than 1 minute. Drain off most of the aqueous layer, wait 30 seconds, drain off the rest of the aqueous layer formed and a little of the organic layer into a beaker. This is to make certain that there is no water in the organic layer. Drain the remainder of the organic layer into a 50 ml beaker containing about 1 g of solid sodium hydrogen carbonate. As soon as the bubbling stops and a clear liquid is obtained, decant it into a clean, dry 50 ml erlenmeyer flask containing a few beads of anhydrous calcium chloride7. Stopper the erlenmeyer flask, label and turn in to the Instructional Assistant or Instructor for Gas Chromatograph Analysis.
D. Gas Chromatography:
With the help of the Instructor or Instructional Assistant, obtain a gas chromtogram of the products from the reaction of n-butyl alcohol and t-butyl alcohol.
THE FOLLOWING POINTS MAY BE HELPFUL IN INTERPRETING YOUR RESULTS:
1. The retention time of similar compounds are usually in the order of increasing boiling points.
The sensitivity of a thermal conductivity detector, such as used in the instrument employed for this experiment, is very nearly the same for comparable compounds.
2. The quantity of a substance present is directly proportional to the area under the peak caused by that substance on the chromatogram so the percent composition can be approximated by comparing relative peak areas. A convenient way to meaure the area is by geometric approximation, or triangulation. Measure the height of the peak above the baseline (h) and the width of the peak at one-half the height (w1/2) and use the equation A = h x w1/2.
POST WRITE-UP - ANALYSIS
Comment specifically on the following: a. Which halide predominated in each reaction. b. The relative quantities of the halides in each reaction, based on the percent of each produced as calculated from the peak areas. c. The relative rates of nucleophilic attack by the bromide ions
and chloride ions on the two alcohols at these temperatures10, based on the ratio calculated from the peak areas. d. The relative reactivity of the two alcohols, based on the reaction conditions.
NOTES
1. This medium will be used for both the n-butyl alcohol and the t-butyl alcohol.
2. Ammonium salts are used because both are soluble at the concentration needed for this conversion.
3. If the solution is cooled too much, the ammonium salts may start to crystallize out. Allow the mixture to stand on the heat bath until your reflux apparatus is set up and then allow to cool. It may be necessary to reheat the mixture if crystallization occurs.
4. If the t-butyl alcohol reaction is not done during the same lab period, transfer the 35 ml of solvent-nuclophile medium to a 125 ml Erlenmeyer flask, cork and store.
5. Violent boiling will cause loss of product. A small amount of hydrogen chloride gas may be emitted from the condenser, this does not indicate a loss of product.
6. Be careful not to shake the hot solution as you remove the heating mantle or a violent boiling and bubbling action will result, causing loss of material out of the top of the condenser.
7. Enough anhydrous calcium chloride should be added so that the beads are free flowing.
8. There will be a lot of pressure build up from the mixing of the warm liquids. Frequent venting is necessary to prevent blow out of the reaction mixture.
9. Any solids that were originally present in the separatory funnel should dissolve during this period.
10. In this experiment any exchange of halogen (i.e., attack of bromide ion upon alkyl chloride and vice versa) is negligible, because the alkyl halides formed are insoluble in the reaction mixture and thus are not in the same phase as the ions.
Partial Flow Sheet
1-Butanol Reaction NH4+ , Cl-, Br-, H2SO4, H2O, H3O+ , HSO4- 1-butanol, n-butyl bromide, n-butyl chloride, 1butene separate
U---------------U---------------U aqueous layer organic layer
wash H2O / separate
U-------------U------------U aqueous layer organic layer wash NaHCO3 / separate
U-----------U---------U
NaHCO3 layer organic layer
Na2SO4 / decant
U----------------U----------U solid liquid
CaCl2
U-----------------U------U solid liquid
Partial Flow Sheet t-Butyl Alcohol
NH4+ , Cl-, Br-, H2SO4, H2O, H3O+, HSO4- t-butyl alcohol, t-butyl chloride, t-butyl bromide,
1-methyl-2-propene separate layers
U-----------U-----------U aqueous layer organic layer
NaHCO3 / decant
U-------------U-----------U solid liquid
CaCl2
U--------------U-----------U
I solid liquid
TPS Revised 7/03
Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | Experiemnt 5 | Experiment 6 | Experiment 7 | Home
©2003 Terrence P. Sherlock