- UTS Chemistry Review
advertisement

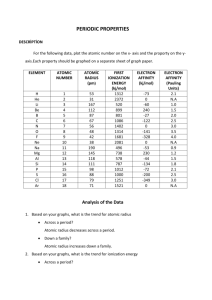
GUIDE TO EFFECTIVE WRITING UTS C HEMISTRY R EVIEW INTRODUCTION Welcome new contributors to UTS Chemistry Review! This post will go over the basics of putting together an article. It provides some general guidelines for structure, factual accuracy, visuals, and writing style. In addition, an exemplar is provided at the end to serve as a template or reference for your own articles. If you have any questions, feel free to email jeff.an@utschools.ca or alex.cui@utschools.ca. As you browse through these recommendations, you should keep a few things in mind: The overall purpose of writing is to communicate effectively To do so, you must find a way to engage your readers The manner in which you write will inevitably affect your ability to captivate your audience Every writer must master the basic elements of effective writing (structure, grammar, tone, etc.) before adopting a “personal” style Effective writing can only come with persistent practice! WRITING GUIDELINES STRUCTURE An introduction, a body, and a conclusion is mandatory o Clearly state the topic in the introduction, along with the purpose of the article (are you explaining a concept, discussing an opinion, etc.) o Elaborate upon the topic in your body o Dedicate at least two paragraphs to your body section o Summarize your main points in the conclusion; you may also want to discuss real-world applications here, connecting your article to the “bigger picture” Your body paragraph should follow a clear sequence of thought such as: o Chronological order o Broad description progressing to specific ideas o Categorical breakdown of a topic o Explanation of a concept followed by an example question o Any other sequence of thought, provided that it is logical and easy to follow Limit each paragraph to a maximum of 6 or so sentences; larger blocks of text can be discouraging FACTUAL ACCURACY Cite all sources in the APA style* (click the link for the APA style guide) In-text citation should follow the format (author, date); in the case that the author is mentioned in the text itself, simply include the (date) at the end of the phrase Both Microsoft Word and Google Docs can complete your references section for you o Simply click “Insert citation” under the References tab In the APA style, the list of sources at the end of the article is called “References” o All sources listed in the References section must correspond to one or more in-text citations in your article o The same list is often referred to as “Works Cited” in MLA style *In its infancy, UTS Chemistry Review did not require citations as the medium was closed to the public. Moving forward, factual information must be cited in accordance with the guidelines above. Previous posts will be updated over time to adhere to the new format. In contrast, a bibliography contains all works cited, consulted, or even simply referred to (not required for UTS Chemistry Review) Citations should be provided for all factual information relating to your topic that is not generally accepted o For example: A study conducted by Mahadevan (2016) shows that… Arctic ice cap volume has decreased by 11% over the past 5 years (WHO, 2013). The atmosphere of the Earth is mostly nitrogen. Chemistry is my favorite subject at school. o Essentially, cite everything that does not immediately appear from a Google search with many similar results (Fig. 1) o Fig. 1: Example of when you do not need to cite something: example of when a caption is not necessary VISUALS Visuals can be an excellent way of clarifying information All visuals must serve a clear purpose, such as illustrating a trend or presenting results All visuals (tables, graphs, etc.) must be referred to in the actual text Captions are not necessary for visuals that do not require further explanation (see Fig. 1) All created graphs should follow the UTS “Guide to Graphs”, which can be found here All visuals not created by the student must be cited using APA format in a note below the visual o Example: Adapted from The Cuspidor, p. 107, by Chee J., 2010, University of Toronto Schools Press. o The full citation must also appear in the References list WRITING STYLE Steer away from long, winding, densely-packed sentences brimming with unnecessary adjectives, which undeniably distract the reader from the purpose of the sentence, and furthermore, do not pack an excessive number of independent clauses into one sentence Include some kind of hook in your introduction, if possible o For example: In a single second on August 6th, 1945, thousands of souls were silenced. This is the power of the atomic bomb… Vary sentence length and construction when possible. Short sentences can get boring. A lack of pauses can contribute to monotony. Use a comma, save a paragraph. Use semi-colons to introduce independent clauses that follow logically from the previous phrase o Example: I am hungry; I haven’t eaten since Monday. Use colons to introduce a list or an independent clause that expands upon the previous sentence o Example: I found several things today: x, y, velocity, acceleration, and even time. The teacher made an announcement: “The laboratory exam is cancelled.” Try not to overuse words such as “thus”, “therefore”, “hence”; these are intended for indicating logical sequences of thought and not for simply sounding professional Use the active voice instead of the passive voice whenever possible o For example: Do: “I injected 10mL of HgCl2 into the patient” Don’t: “10mL of HgCl2 was injected into the patient” Feel free to use questions to occasionally spark interest or create suspense (e.g. What could explain this unexpected result?) Bold or underline phrases that you want to stress, but make sure that you are consistent (don’t use bold font and underlined text interchangeably) EXEMPLAR In the interest of space, I have chosen one of the shorter posts on UTS Chemistry Review. Note that your articles will not have to be as explanatory as this one. Any rule has exceptions, and periodic trends have many. In this post we discuss the most important periodic trend deviations. E LECTRON AFFINITY Recall that electron affinity (EA) is the energy released upon addition of an electron to a neutral atom. As we discussed, electron affinity usually increases from left to right across a period since the electronegativity of the atoms in question increases. In other words, across a period, the ability of the atoms to attract an electron increases and more energy is released when an anion is formed. Compare these two examples: Li + e– -> Li– process) F-+ e– -> F– ∆H = -59.6 kJ/mol (a low amount of energy is released in this relatively unfavourable ∆H = -328.16 kJ/mol (a high amount of energy is released in this highly favoured process) However, there are two important exceptions to this trend. Firstly, contrary to what would normally be expected, fluorine has a lower electron affinity than chlorine, even though it has a higher electronegativity. Why is this the case? The answer lies in the small size of the fluorine atom compared to the relatively large chlorine atom. Thus, “squeezing” an extra electron into the fluorine valence is energetically more demanding and less favourable than adding an electron into chlorine’s outer shell. Of course, the other larger halogens (Br, I, At, etc.) all have lower EAs than fluorine since they have substantially lower electronegativities. It is only in the case of chlorine, which has a similar electronegativity but much larger size, that this exception occurs. For similar reasons, oxygen also shows a lower electron affinity than sulfur. Energetically, it is simply more favourable to form a sulfur anion than it is to form an oxyanion. I ONIZATION ENERGY Figure 1 displays the first ionization energies of period 3 elements, showing a general increase riddled with a few "dips". Why do these dips occur? Unfortunately, a concrete explanation of these exceptions requires an understanding of quantum mechanics, although the exceptions can be summarized as follows: elements in group 3 and 6 display lower than expected first ionization energies. For those already well-versed in quantum mechanics, the explanations follow: Figure 1: Ionization energies of the Period 3 elements. Reprinted from “ Trend in electronegativity of Period 3 Elements” by Creative Chemistry, n.d., retrieved from http://www.creative-chemistry.org.uk/alevel/modu le1/trends7.htm Magnesium to aluminium Consider the electron configurations of Magnesium and Aluminum: Mg: 1s2 2s2 2p6 3s2 Al: 1s2 2s2 2p6 3s2 3p1 The outer electron in aluminium is in a p sub-level. This is higher in energy than the outer electron in magnesium, which is in an s sub-level, so less energy is needed to remove it. Phosphorous to sulfur Consider the electron configurations of phosphorus and sulphur (Fig. 2): Figure 2: Electron configurations of phosphorus and sulphur. Reprinted from “First ionisation energies of Period 3 elements” by Creative Chemistry, n.d., retrieved from http://www.creativechemistry.org.uk/alevel/module1/trends6.htm The 3p electrons in phosphorus are all unpaired. In sulphur, two of the 3p electrons are paired. There is some repulsion between paired electrons in the same sub-level. This reduces the force of their attraction to the nucleus, so less energy is needed to remove one of these paired electrons than is needed to remove an unpaired electron from phosphorus. As you have seen, there are a few interesting exceptions to periodic table trends that occur in ionization energy and electron affinity. While these deviations may seem trivial, they can actually contribute to very important effects. For instance, hydrofluoric acid (HF) is classified as a weak acid: that is, it only partially dissociates in water. Why is this the case? Since fluorine has a lower than expected electron affinity, fluorine atoms in HF molecules are unwilling to accept electrons from the hydrogen atoms. Thus, dissociation of HF into H+ and F- does not occur appreciably. Clearly, the exceptions to the trends matter. WORKS CITED Creative Chemistry. (n.d.). Ionization energies of the Period 3 elements [Graph]. Retrieved October 23, 2015, from http://www.creative-chemistry.org.uk/alevel/modu le1/trends7.htm Creative Chemistry. (n.d.). First ionisation energies of Period 3 elements [Graph]. Retrieved October 23, 2015, from http://www.creative-chemistry.org.uk/alevel/module1/trends6.htm