File
advertisement

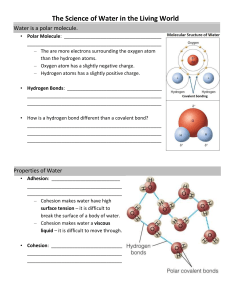
WILEYPLUS ASSIGNMENT #3 DNA forms a double-stranded helical structure that contains base pairs whereas RNA is single stranded and does not form base pairs or adopt a double helical conformation. FALSE Phosphodiester bonds join the 3' hydroxyl of one nucleotide to the 5' hydroxyl of the next. The complementary base paired double helix structure of DNA is ideal for replicating genetic information because each DNA strand can act as a template for the synthesis of its complementary strand with the hereditary information encoded in the sequence of bases on either strand. Alterations to the genetic material of an organism are known as mutations The template for protein synthesis is known as messenger RNA. DNA is the carrier of genetic information in all cells and in many viruses. The central dogma of molecular biology asserts that DNA directs its own replication to produce new DNA molecules; DNA can also be translated into protein. A nucleotide can be called a nucleoside-5'-phosphate. Cytosine, uracil, and thymine are common pyrimidines. An important property of many biological polymers is that information is contained in the sequence of its residues. The linkage between individual nucleotides in a polymer of DNA is known as a phosphodiester bond. An A:T base pair is stronger than a G:C base pair due to an extra hydrogen bond. The synthesis of a complementary strand of RNA from a DNA template strand is known as transcription . The synthesis of a protein from a messenger RNA template is known as translation . The ribosome is the organelle that is the site of protein synthesis. The alpha-carbon is the carbon adjacent to the carbon of the carboxyl group. Polyunsaturated fatty acids contain multiple double bonds. Fatty acid double bonds almost always have the cis configuration. Lipid molecules are NOT the only essential components of biological membranes. Proteins are also essential components of biological membranes. The chemical structure of Vitamin D is closely related to cholesterol. Cholesterol contains an alcohol group, an octyl group, and is rigid. However, the ring structures are NOT aromatic. In mammals, cholesterol is the metabolic precursor to steroid hormones. Glycerophospholipids are derivatives of glycerol-3-phosphate. The primary biological functions of lipids are to serve as signaling molecules, serve as components of biological membranes, and to serve as energy stores. WILEY PLUS ASSIGNMENT #4 Noncovalent associations between neutral molecules are due to forces known generally as London dispersion forces. Hydrogen bonds are an example of weak non-covalent interactions, which are common in biological molecules. Water is considered polar because the oxygen atom carries a partial negative charge of -0.66e, while the hydrogen atoms are each partially positive, +0.33e. Water solvates both positive and negative ions. Osmosis is the movement of solvent across a semipermeable membrane from a region of high solvent concentration to a region of lower solvent concentration. The hydrophobic effect is the tendency of water to minimize its contacts with hydrophobic molecules. Amphiphiles or amphipathic molecules are molecules that have both polar and nonpolar segments. -CH3 lacks polarity and therefore cannot make a hydrogen bond to water. Nonpolar substances have low solubility in water because of their inability to form sufficient hydrogen bonds with water leading to their exclusion from solution. The negative of the log[H+] is defined as pH . Acids that only partially ionize are called weak acids. The buffering capacity of a weak acid is greatest when pH = pKa. The concentration of hydrogen ions at pH 3 is 10-3 M because pH is equal to -log[H+]. The pK of acetic acid is 4.76. At pH = 5, [(deprontonated)A-] > [(protonated)HA]. The buffering range is pK ± 1. Ions that are surrounded by layers of solvent are said to be solvated . The tendency of water to minimize its contacts with hydrophobic molecules is termed the hydrophobic effect . A van der Waals interaction between two methyl groups is the weakest attraction. Water can solvate both positive and negative ions because the negative oxygen atom interacts with positive ions, and the positive hydrogen atoms interact with negative ions. Strong acids, such as HCl, transfer all of their protons to H2O. A buffer is a solution that resists pH changes. The pK of ammonia is 9.25. At pH = 7, [NH4+] > [NH3]. Hydrophobic interactions between nonpolar molecules or groups require the presence of surrounding water molecules. The strongest non-covalent interactions are ionic interactions. The pH at the midpoint of an acid/base titration is equal to the pK of the corresponding acid. WILEYPLUS ASSIGNMENT #5 Choose the correct answer from the list. Not all the answers will be used. When a peptide bond is formed, an alpha-amino group reacts with a carboxylate group. In the peptide Trp-Ser-Val, valine is at the C-Terminus. At a pH above its pK, the phenolic group of tyrosine is deprotonated. At a pH below its pK, the ɛ-amino group of lysine is protonated. In the tripeptide Trp-Val-Phe, the N-terminal residue is tryptophan. In the tripeptide Lys-Pro-Ile, there are three charged groups at pH 7. Threonine has an uncharged polar side chain at pH 7. At pH 1, lysine (pKs are α-carboxylate 2.16, α-amino 9.06, ɛ-amino 10.54) would be charged as follows: 0 α-carboxylate, +1 α-amino, +1 ɛ-amino, +2 net charge. All the standard amino acids except Glycine are optically active because it has two –H attached. Both methionine and cysteine contain sulfur. Threonine has an -OH group that can hydrogen bond. The side chain of methionine (-CH2-CH2-S-CH3) is not aromatic. The side chain of Isoleucine is considered nonpolar. The primary structure of a protein is the amino acid sequence of its polypeptide chain, or chains if the protein consists of more than one polypeptide. The local spatial arrangement of a protein's backbone atoms without regard to the conformations of its substituent side chains is called its secondary structure. Nonpolar residues occur mostly in the interior of proteins. The entire three-dimensional structure of a single-chain protein, including that of its side chains is called its tertiary structure. The hydrophobic effect is the main determinant of protein stability and a protein folding into native 3D (tertiary) structure. Hydrogen bonding and ion pairing contribute relatively little to a protein’s stability. Two amino acids of the standard 20 contain hydroxyl-groups. They are threonine and serine. The only difference between Gly and Ala is the replacement of a hydrogen atom in Gly by a methyl group in Ala. Hence, Gly and Ala have similar structural properties. Therefore Ala is MOST likely to be a conservative substitution for glycine. The primary driving force in the formation of protein tertiary structure is the exclusion of non-polar substances from aqueous solution. .The hydrophobic effect is the major factor determining the stability and structure of native proteins. Hydrogen bonds stabilizes the folding of a polypeptide backbone into regular secondary structure. Glu-Asn-Ser-Thr-Gln would most likely be located on the surface of a soluble globular protein because All five of these amino acids are polar. Quaternary structure is stabilized by the same types of noncovalent interactions as tertiary structure.