211-13Acid-Base-8 - Moravian College Chemistry Department
advertisement

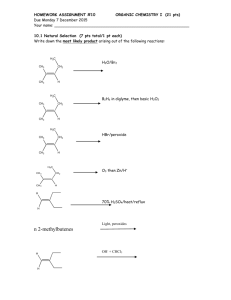
Chemistry 211 Fall 2013 Equilibrium Controlled Reactions: Acid-Base & Electron Energy-8 A. Comparison of the Magnitudes of Structural Effects on HEE Energies Based on our studies of acid-base reactions we have concluded that: The pKa of an acid is determined by the energy of the conjugate base. (Relative Effect Assumption) The highest energy electrons (HEE) are the ones that determine the site of reaction of a structure (Relative Effect Assumption) We can use the pKa of the conjugate acid (pKaH of the conjugate base) as a measure of the energy of the HEE on a conjugate base. Figure 1 below provides structures of conjugate bases with their pKaH values. These have been taken from Tables A-E in acid-base-3-7 activities. Use these data and the following questions to explore the relative magnitudes of the five effects we have conceived. 1. Circle highest energy electrons on each of the structures in Figure 1. Conjugate Base ~37 Conjugate Base CH3 CH2: - CH3 O: ~37 16 CH3 CH2 ~42 CH2 CH ~40 HC C Conjugate Base pKaH - CH2: - CH2 : 25 - 16 - -1 O: O: H Figure 1: Various Structural Effects on Electron Energies ~ 50 41 16 10 Conjugate Base :O: pKaH - 4.8 CH3 C O: :O: - 3.7 H C O: :O : F3C C : : CH3 O CH3 NH - pKaH : : O: 10 CH3 ~42 Conjugate Base : : - : : CH3 H N H : : : : H pKaH Inductive : : : : CH3 N pKaH Delocalization Hybridization Nuclear Charge Net Charge - 0.0 O: 2 Acid-Base & Electron Energy- 8 2. Estimate the energy differences (in pK units) between the HEE on each pair of structures below: DG pK units vs. CH3 CH2: - H H CH3 O H CH3 CH3 vs. CH3 NH - vs. CH3 H N H vs. CH3 O NH - CH3 CH2 vs. CH2 CH CH2 CH vs. HC C O: : : O: : : CH3 H - CH3 N : : : : vs. CH3 N DG pK units DG pK units H DG pK units DG pK units O: 3. Below, list the effects in 2 in order of decreasing G: - O: vs. :O: CH3 C O: H C :O: vs. F3C C - O: : : vs. - CH3 C : : O: CH2 : : : : : - vs. :O: :O: : : CH2: - : : - - O: Acid-Base & Electron Energy-8 3 B. Use of empirical benchmarks for competing structural effects on electron energies: These and future applications require some empirical evidence, which provides benchmarks for relating effective nuclear charge (C vs. N vs. O), net charge (- vs. neutral) and carbonyl delocalization effects when they do not reinforce each other. These pKaH values are ones that you must KNOW. Conjugate Base pKaH Conjugate Base pKaH : : O 15.7 Isolated electrons on : CH3 Delocalized carbanion electrons alpha to C=O 10 NH2 O: - - : : :OH 20 : : : : - - : : C CH2 : CH3 O 10 Delocalized O electrons alpha to benzene ring O Isolated electrons on neutral N CH3 C O: Delocalized O 4.8 alpha to C=O electrons For each of the following reactions: Circle the HEE in the reactants and products. Use the change in energy of the highest energy e-'s (HEE) to predict whether the equilibrium constant should be > or < 1. Provide a warrant indicating how you developed your claim. Answer the reflection question for each. 1. CH3 NH3 + H2O OH + CH3 NH2 Warrant: Reflection: What conclusions can you draw about the relative energies of a lone pair of electrons isolated on a neutral nitrogen atom vs. one isolated on a negative oxygen atom? + CH3 NH2 : : : : O O : : : 2. CH3 C Warrant: H : : O CH3 C O : - + + CH3 NH3 4 Acid-Base & Electron Energy- 8 Reflection: What conclusions can you draw about the relative energies of a lone pair of electrons isolated on a neutral nitrogen atom vs. one delocalized over two carboxylate oxygen atoms? : : : : 3. O CH3 C Warrant: O CH3 + H2O OH + CH2 C CH2 Reflection: What conclusions can you draw about the relative energies of a lone pair of electrons isolated on a negative oxygen atom vs. one delocalized over an -carbanion system? C. General applications of electron energies to organic reactions. For the following pair of equilibrium controlled reactions use the change in energy of the highest energy e-'s to predict which reaction should produce more products at equilibrium. Provide a warrant indicating how you developed your claim, being sure to identify the HEE of the reactants and products of each reaction. Illustrate the relative energy changes using the Rxn. Coordinate Diagram provided. O CH3 CH2 C + O NH CH3 CH2 + C G OH O CH3 CH2 C O N N + CH3 CH2 NH + OH C Reactants Rxn Coord Products Acid-Base & Electron Energy-8 5 Out of Class Applications for Acid-Base-8 A. Reading Assignment: CGW Chapter 8 pp. 161-181 B. Problems Claden, Greeves & Warren Website (http://www.oup.com/uk/orc/bin/9780199270293/) End of chapter questions: Chapter 8 questions 1-8 C. Assignment: 1. On p. 167 CGW discusses the preferred form of amino acids (the building blocks of proteins) in water. They indicate that the predominant form of amino acids is the zwitterion form rather than the amino-carboxylic acid form. Provide a warrant based on the HEE of each amino acid form and the benchmark pKas introduced in Acid-Base-8 that accounts for the results stated in CGW. 2. Analyze the structures of each of the following compounds, then circle the most acidic proton(s) and provide a warrant indicating how you developed your claim. Your warrant must be based on the theories of the effects of structure on e- energies developed in class. H H a. H H H H b. H C O C c. H2N H OH d. H2N NH3 H C O C C C CH2 CH3 H C H H H 3. For each of the following acid-base reactions use the change in energy of the HEE to predict whether the equilibrium constant should be > or < 1. Provide a warrant indicating how you developed your claim being sure to identify the highest energy e-'s of the reactants and products. O a. O C OH + O O C OH b. O + O C O O O C O C O O + OH O + C OH O 6 Acid-Base & Electron Energy- 8 4. For the following pair of equilibrium controlled reactions use the change in energy of the highest energy e-'s to predict which reaction should produce the more product at equilibrium. Provide a warrant indicating how you developed your claim being sure to identify the highest energy e-'s of the reactants and products of each reaction. O CH3 CH2 A O O C O CH3 CH2 CH3 C + O O + O CH2 C O CH3 CH3 CH2 CH2 C C B + O CH3 O CH2 O CH3 CH2 C O + CH3 CH2 O O D. Nomenclature of Aldehydes and Ketones References: 1. CGW: Ch. 2 p. 30-31 2. Tutorials: a. https://chemistry.boisestate.edu/richardbanks/organic/nomenclature/organicnomenclature1.htm Developed by Richard C. Banks, Professor of Chemistry, Boise State University Provides questions with answers Sections Aldehydes Ketones b. http://elchem.kaist.ac.kr/jhkwak/OkanaganPdb97/nomenclature/index.htm Developed by Professor emeritus Dave Woodcock at Okanagan College of the University of British Columbia, Canada (Contains many examples.) Sections: 6. Functional Groups with Suffix and Prefix IV. Alkanones (Ketones) V. Alkanals (Aldehydes) Acid-Base & Electron Energy-8 7 c. http://www.acdlabs.com/iupac/nomenclature Developed by Advanced Chemistry Development Laboratories (Gives detailed IUPAC rules for nomenclature.) Recommendations 1993 R-5 Applications to Specific Classes of Compounds R-5.6 Aldehydes, Ketones, their Derivatives and Analogues R-5.6.1 Aldehydes, thioaldehydes, and their analogues R-5.6.2 Ketones, thioketones, and their analogues. 3. Applications a. Name the following: O O O O O b. Draw structural formulas for the following compounds: 3-phenyl-2-pentanone 4-bromo-3-ethyl-5-heptenal 3-cyclopentenone 4-methoxybenzaldehyde