NuclearDecay_Printable
advertisement

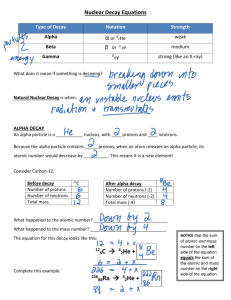
Chem 112 Class Guide: NUCLEAR DECAY Chapter 21, Sections 1, 2 and 3 Learning Goals: Upon completion of Chapter 19, Sections 1-4, you should be able to determine the following: Identify α, β, and ϒ particles Predict the products of α, β, ϒ, positron decay or electron capture reaction Chapter reading Guide: Chapter 21, Sections 1, 2 and 3 Section 1: Radioactivity Read Chapter 21.1 Before we begin, it might be a good idea to review Chapter 2 Section 3 about isotopes. Remember that 146C has a mass number of 14 (neutrons plus protons) and an atomic number of 6 (number of protons). You should be able to determine that this isotope of carbon has 8 neutrons. Also, we could write this as carbon-14 instead of 146C . A nuclide is a nucleus containing a specific number of protons and neutrons. This is another way of saying isotope. While the vast majority of nuclides are stable, radionuclides are unstable and spontaneously emit particles and/or electromagnetic radiation. The emission of particles is called nuclear decay. There are 5 types of nuclear decay: 1.) Alpha Decay Occurs when a radionuclide emits an alpha particle: U 238 92 Th 24 (Note that the mass number and atomic numbers on both sides of 234 90 the equation are equal, or 238 = 234 + 4 and 92 = 90 + 2.) 2.) Beta Decay Occurs when a radionuclide emits a beta particle: 131 53 0 I 131 54Th 1 (Again, the mass numbers and atomic numbers on both sides of the equation are equal. This will be true for all types of decay.) 3.) Gamma decay Occurs when a radionuclide emits a gamma particle: U 00 U 238 92 238 92 4.) Positron Emission This decay occurs when a radionuclide emits a positron. A positron is like an electron, but it has a positive charge instead of a negative charge. C 115 B 10 e 11 6 5.) Electron Capture Not technically a decay, as the reactant captures an electron 81 37 Rb 10 e 3681Kr Table 21.3 summarizes these reactions. Example: What particle is produced when plutonium-242 decays to uranium-238? We need the atomic numbers and the mass numbers to equal on both sides of the arrow, so we need all the information about the nuclides to get a rough idea of where to begin (use the periodic table for this): 242 94 Pu 238 92 U The mass number of Pu is 4 greater than that of U and the atomic number of Pu is 2 greater than U. An alpha particle has a mass number of 4 and an atomic number of 2, so we need an alpha particle on the products side of the equation to balance: 242 94 Pu U 24 238 92 Try practice exercises 21.1 and 21.2 Section 2: Patterns of Nuclear Stability Read Chapter 21.2 It is possible to predict the type of the type of decay a nuclide will go through by looking at the ratio of neutrons to protons and looking at the “belt of stability” (figure 21.2) Nuclei with a higher than “normal” neutron-to-proton ratio will likely decay via beta emission. Nuclei with a lower than “normal” neutron-to-proton ratio will likely decay via positron emission OR electron capture Nuclei with 84 or more protons are likely to decay via alpha decay Example: Predict the type of radioactive decay iodine-120 undergoes. To do this, we need to calculate the neutron/proton ratio. neutrons (120 – 53). The ratio is: 120 53 I has 53 protons and 67 67 1.26 . This ratio is higher than normal (>1.00) so will 53 likely undergo beta emission: 120 53 I 10 e 120 52Te Remember that when the number of protons changes (goes from 53 to 52), the identity of the element changes. Nuclides that have an even number of neutrons, protons or both are more likely to be stable than nuclides with odd numbers of protons and/or neutrons. Also, elements with full shells are more likely to be stable than nuclides with other numbers of protons and/or neutrons (full shells are: 2, 8, 20, 28, 50, 82 and 126). Try practice exercise 21.3 Section 3: Nuclear Transmutations Read Chapter 21.3 A nucleus can change if it is struck by a neutron or another nucleus. Example: Write a balanced equation when 14 7 N reacts with an alpha particle to produce 116C and a proton. 14 7 N 24 116 C 11H Remember that the atomic numbers and the mass numbers have to equal on both sides of the arrow! We can write the reaction above another way: 14 7 N , p 116 C . The nucleus on the left is the reactant ( 147 N ), the item on the left inside parentheses is the particle that struck the reacting nuclei (alpha particle), the item on the right inside parentheses is the particle that is released (p, or proton), and the nuclei on the right is the nuclei that is formed in the reaction. Some of the abbreviations you will see: Abbreviation Particle 4 α 2 β 0 1 γ 0 0 n 1 0 p 1 1 n H Try Practice exercise 21.5 Learning Resources Chapter Learning Goals Chapter 20, Sections 1, 2 and 3 Learning Goals Pre Class Assignment: This assignment must be completed prior to the next class. Check your syllabus for the exact due date and time. Complete to the pre class assignment (http://berks.psu.edu/clt/chem112/NuclearDecay_HW.docx) Submit a copy to the dropbox located in ANGEL called “Pre Class Assignment Submission: Nuclear Decay” End of Chapter Problems: Practice with these problems if you are having difficulty with any of the concepts covered in this class guide AFTER we have met in class. If you cannot easily complete these problems, seek help from your instructor, your mentor or the learning center Chapter 21: 7, 9, 13, 17, 27