Catalyst - Introduction
advertisement

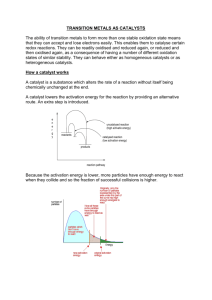
The Firecat® catalytic combustor What is catalysis? Catalysis is a process in which the rate of a chemical reaction is changed by a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. Although catalysts are not consumed by the reaction itself, they may be inhibited, deactivated or destroyed by secondary processes. In heterogeneous catalysis, typical secondary processes include coking where the catalyst becomes covered by polymeric side products. Catalysts do not change the extent of a reaction: they have no effect on the chemical equilibrium of a reaction because the rate of both the forward and the reverse reaction are both affected (see also thermodynamics). The fact that a catalyst does not change the equilibrium is a consequence of the second law of thermodynamics. Suppose there was such a catalyst that shifted equilibrium. Introducing the catalyst to the system would result in reaction to move to the new equilibrium, producing energy. Production of energy is a necessary result since reactions are spontaneous if and only if Gibbs free energy is produced, and if there is no energy barrier, there is no need for a catalyst. Then, removing the catalyst would also result in reaction, producing energy; i.e. the addition and its reverse process, removal, would both produce energy. Thus, a catalyst that could change the equilibrium would be a perpetual motion machine, a contradiction to the laws of thermodynamics. (http://en.wikipedia.org/wiki/Catalysis) Though catalysts are used in countless applications in chemistry, food processing, painting, coating, laminating, engine emission controls...; we’re interested in the small group of so-called “Oxidation Catalysts” only. Oxidation catalysis works as follows: O2 in a first step dissociates to 2O which is then forming unstable chemical compounds with noble metal particles incorporated in a wash-coat layer. These precious metals are Palladium (Pd), Platinum (Pt), and Rhodium (Rh). By this way residual oxygen that would otherwise just leave thru chimney is held back and thus forming an environment with “extra oxygen”; hot, highly reactive, “free” oxygen radicals. Since bonding forces between O and noble metals are very low (which is in fact the reason why these metals are “noble”), O can easily dissociate with the metal and bond with higher reactive compounds such as CO: On the other hand: since oxidation by free oxygen radicals is the cause of its effectiveness, one can understand why noble-metal catalysts are oxidizing more or less any “burnable” chemical compound; although at different rates and temperatures… … leaving CO2 and H2O (vapour) as waste gas: We have now already talked about the effectiveness of the cat – now we’ll see how it is made: we’re using cordierite ceramic as substrate with a washcoat to carry precious metal particulates. Cordierite ceramic is characterized by high porosity: which makes it easy to coat and enhances the surface area for maximum roughness (surface area) low thermal expansion: cordierite has a near zero thermal expansion which makes it very stable and not prone to sloughing off the coating. The surface area of even the best ceramic carrier is not very high. One way to increase surface area is the shape (honeycombs) which are derived by cold extrusion. But any ceramics surface is sometimes compared to the smooth side of a glass shower door. If you splash water onto a level, smooth plate of glass the water will puddle into a few large puddles. This is because it has a small amount of surface area. We want a high surface area like the rough side of a shower door. The water when splashed onto the rough side will tend to spread out giving the maximum surface. Another analogy is that we are trying to create a Thomas's English Muffin where there are lots of nooks and crannies. The way we do this is by application of a wash coat or surface primer. The most common type is gamma alumina. Alumina has many phases but the one with the highest surface area is gamma. One of the reasons why it is recommended that the catalyst be kept below 815°C and out of high temperature and direct flame is that gamma alumina at high temperatures will convert to alpha alumina and lose much of its surface area; thus greatly reducing catalytic activity. The gamma alumina is applied as slurry and then calcined to fix it. This tremendously increases surface - approaching 2,000 times the surface area of the bare substrate - is now ready for the catalyst. The most common catalysts are from the transition group metals. In this we have base metals and precious metals. Our choice has been to use the precious metals platinum and palladium. These metals are excellent oxidation catalysts and are very stable and long lived in the application we have selected. The metals are put into a solution and are applied in a variety of methods from spray, to pump to dip coating. The technique is not as important as the controls to assure the loading of the metal is not too heavy or too light and is consistent from part to part. Too much metal is a waste of money as the diffusion controlled process of catalytic oxidation is determined by the relative factors of time, temperature and turbulence; the “3Ts” determining any kind of burning. When the correct amount of metal is used, extra metal does not influence efficiency as much as it will influence life, especially in an abusive application. After application, the metal coated parts are calcined again to affix the metal onto the surface area. Cordierite ceramic with washcoat and precious metal coating. Red-hot glowing cat in action.