Nick`s-Essential+Biology+03.1+Chemical+Elements+and+
advertisement

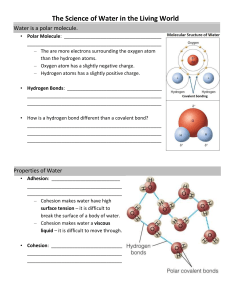
Essential Biology 03.1: Chemical Elements and Water 1. What are the four most commonly occurring elements in living things? Hydrogen, Oxygen, Carbon, Nitrogen 2. State some functions of each of these elements in living things: a. Iron Symbol: Fe Ion: 26 Function(s): used to make chlorophyll , used in photosynthesis as ferredoxin. It also facilitates the movement of electrons in cells. a. Sulphur Symbol: S Ion: 16 Function(s): It is a reactant for chemosynthetic bacteria (chemoautotrphs). b. Calcium Symbol: Ca Ion: 20 Function(s): It is used in the structure of bones and teeth in animals and can also be used for blood clotting. c. Phosphorous Symbol: P Ion: 15 Function(s): It is essential for the formation of the phospholipid bilayer. They are also the active components in ATP molecules and make up part of the backbone of DNA. d. Sodium Symbol: Na Ion: 11 Function(s): Sodium Is essential in generating an action potential for nerve impulses. e. Potassium Stephen Taylor Symbol: K Bandung International School Ion: 19 http://sciencevideos.wordpress.com Essential Biology 03.1: Chemical Elements and Water Function(s): Potassium also plays a role in nerve impulses and has a strong influence in osmosis. 3. In the space below, draw three water molecules attracted to one another by hydrogen bonding. Include labels to show the polarity of the molecules. Annotate: How does H-bonding work? H Oxygen H H Oxygen H Oxygen Hydrogen bonds H H 6. Water has many properties which are essential for life. Complete the table below. Thermal Properties Cohesion Stephen Taylor Explanation Significance to living things Water has a high specific heat capacity. This means it takes a lot of energy for the temperature of water to change. This is because there are so many H-bonds. Most organisms are adapted to a narrow range of conditions. The slow heating and cooling of water are ideal for these organisms – there is less risk of extreme changes. A single hydrogen is not very strong. A large number of hydrogen bonds is very strong. Molecules of water stick to each other, and water stick to other surfaces. These properties lead to capillary action, where water will move up xylem against gravity, and surface tension, where the surface of water is strong enough to support insects ad causes drops to form. Bandung International School http://sciencevideos.wordpress.com Essential Biology 03.1: Chemical Elements and Water Solvent Properties Water is a good solvent because it is a polar molecule. It will dissolve polar solutes easily. Polar attractions cause water molecules to surround and isolate the solute molecules. (include uses as a coolant, medium for metabolic reactions, transport medium) Stephen Taylor Bandung International School http://sciencevideos.wordpress.com