Water: Adhesion Property
advertisement

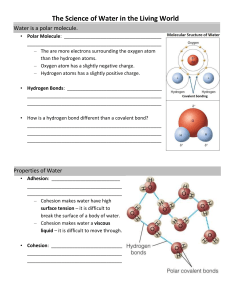
Properties of Water Chapter 2 Water: Adhesion Property Adhesion: an attraction between molecules of different substances Capillary action – water tends to climb up straws, or similar (glass) Meniscus- water makes a curve when sitting in a graduated cylinder- (glass) Wet jeans Mini-lab: Capillary Action Place the tiny tube in the colored water and watch it climb… Does it end up lower or higher than the outside fluid level? Can you see the meniscus in the graduated cylinder? Water: Cohesion Property Cohesion: attraction between molecules of the same substance Insects can walk on water Water forms droplets on certain surfaces (glass, copper, paraffin) Surface tension Mini-Lab: Quicker Picker upper Pour 5 mL of water on your desk. Take the papertowel and place only the very end in the pool of water. Once it stops climbing, measure how far the water climbed. Wipe up the desk Mini-Lab :surface tension Take a small paperclip and float it in a beaker of water What is the trick to doing this? Mini-Lab: cohesion How many drops of water can you fit on a penny? What shape does the water make? Why does the water eventually spill over? Polar? Water is polar, which means the atoms have slight charges, which makes it attract itself and other substances, too. That bond is a hydrogen bond and it is really weak WHY is water polar? Because of the 8 protons in the nucleus, the Oxygen has a stronger attraction for electrons than hydrogen (in other words they share unequally) Other polar compounds? NH3 ammonia HF hyrdogen fluroride Water is more polar than any other compound so it will tend to dissolve ionic compunds, and other polar substances very well Alcohol is also polar, so it does mix with water, but its has a lower degree of polarity, so it won’t dissolve some things that water can OIL- is non-polar Why does ice float? Why is that soooooo important to our planet? The story: Alcohol doesn’t freeze at the same temperature as water…so why did I get in so much trouble?! Water is an exception to many rules It freezes from the top down It becomes less dense when frozen solid Because the hydrogen bond pattern created “pockets of open space” making it less dense or lighter…see bubbles?? In ice?? It ‘s boiling point is too high for its size It’s freezing point is too low for its size The normal pattern for most compounds is that as the temperature of the liquid increases, the density decreases as the molecules spread out from each other. As the temperature decreases, the density increases as the molecules become more closely packed. This pattern does not hold true for ice as the exact opposite occurs. In liquid water each molecule is hydrogen bonded to approximately 3.4 other water molecules. In ice each each molecule is hydrogen bonded to 4 other molecules. The End…..