(Neem) gum - Springer Static Content Server
advertisement

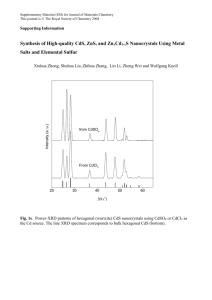
SUPPORTING INFORMATION Biogenic synthesis of Fluorescent Carbon Dots at ambient temperature using Azadirachta indica (Neem) gum Chinmay Phadke$§, Ashmi Mewada$§, Roopa Dharmatti§, Mukeshchand Thakur§, Sunil Pandey§, Madhuri Sharon§€* § N. S. N. Research Centre for Nanotechnology & Bio-Nanotechnology, Ambernath, MS, India € Monad Nanotech Pvt Ltd, A-702 Bhavani Towers, Powai, Mumbai, Ms, India $ Authors have equal contribution *Author for correspondence: sharonmadhuri@gmail.com Phone: +91 9552599207 ___________________________________________________________________________ Stability studies of Azadirachta indica (Neem) gum carbon dots: In order to be used as drug carrier or for any other biological applications, it’s mandatory for CDs to be stable under different circumstances. Stability of CDs was checked at different temperature, pH and in different solvents. 1. Temperature CDs solution was subjected to different temperatures and changes in the optical spectra were measured by UV-Vis spectrophotometer (Perkin Elmer, Lambda 25, USA) at regular intervals. At the different temperatures (4ºC, RT, 37 ºC, 60 ºC and 100 ºC) there was negligible shift (Fig. S1) observed up to 72 hrs indicating exceptional stability making them best candidates for many applications including biological applications. 1 Figure S1. Stability studies of CDs at 4ºC, 37 ºC, 60 ºC, 100 ºC and RT 2. pHStability of 10 ml aqueous CDs solution was checked at both acidic and basic pH. At pH 2, 4, 6 and 8 there was blue shift in the peak (216 to 200 nm) (Fig. S2), which might be because of protonation of NH groups and deprotonation of COOH groups present on the surface of CDs. However blue shift at basic pH 10 and 12 was relatively less (216 nm to 202 nm) may be due to only deprotonation of NH groups. Overall CDs were found to be stable in terms of intensity at all pH conditions. 2 Figure S2. Stability studies of CDs at various pH 3. Solvent stability: Methanol as a solvent shows average stability of C-dots as depicted from the Figure S3. There is a minor blue shift of 2 nm (216 nm to 214 nm) probably due the stabilisation of C-dot surface with addition of hydroxyl groups. On the contrary DMSO and Chloroform show a red shift of 4 nm (216 nm to 220 nm) and 10 nm (216 nm to 226 nm) respectively over increasing period of time in the UV-Vis spectra along with drastic decrease in the intensity (Fig. S3). This could be because of the dampening of the inherent functional groups on the surface of C-dots by interacting with the functional groups of the solvent like –Cl. Interaction with HCl shows a considerable blue shift of 5-6 nm (absorption maxima 210 nm-209 nm) as seen in with a moderate decrease in intensity which remains constant over time. The blue shift and stabilisation could be because of the protonation and deprotonation of amine and carboxylic functional groups respectively as observed and confirmed in the pH stability test for C-dots. C-dots show exceptional stability with acetone with minor blue shift and negligible fluctuation in the intensity 3 as observed in the (Fig. S3). Thus, Acetone, Methanol and HCl and be considered as the most suitable solvents for application of C-dots in drug delivery or bio-imaging. Figure S3. Solvent stability studies of CDs 4