Abstract
advertisement

Keywords:
Dendrimers;
Self-assembly;
Supramolecular chemistry;
Pi interactions;
NMR spectroscopy
Abstract
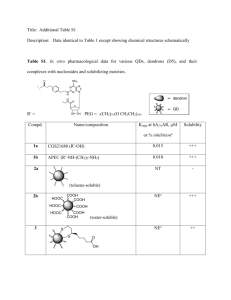
The synthesis of a series of carbosilanes of type 1-Br–C6H4–4-SiMe3–n(CH2CH=CH2)n (n = 1, 2, 3), Si[C6H4–4-SiMe3–
(CH2CH=CH2)n]4, 1-Br–C6H4-4-Si[C6H4–4-SiMe3–n(CH2CH=CH2)n]3 (n = 0, 1, 2, 3), ClSi[C6H4–4-Si{C6H4–4-SiMe3–
n
(CH2CH=CH2)n}3]3, Si[C6H4–4-Si{C6H4–4-SiMe3–n(CH2CH=CH2)n}3]4 (n = 1, 2), and 1-Br–C6H4–4-Si[C6H4–4-Si{C6H4–4-SiMe3–
n
(CH2CH=CH2)n}3]3 (n = 0, 1, 2, 3) is discussed. The structures of Si[C6H4–4-SiMe2(CH2CH=CH2)]4 (7b) and ClSi[C6H4–4-
n
Si{C6H4–4-SiMe2(CH2CH=CH2)}3]3 (10a) in the solid state are reported. The crystal structures of both dendrimers are based on
C–H···πCC (πCC = double bond of the CH=CH2 groups) and π–π interactions. The 3D network structure observed for 7b is
composed of three crystallographically independent head-to-head stacking columns along the crystallographic b axis with π–π
contacts among the C6H4 groups of neighboring molecules, with the columns interacting further by C–H···πCC arrangements. Two
molecules of 10a interdigitate in the solid state to form self-complementary dimers that are stabilized by C–H···πCC and π–π
contacts. The structures of the monomer and the dimer were optimized by using the MM+ force field as implemented in
HyperChem 4.02. In addition, the presence of self-complementary structures in solution is evidenced from 1H NMR spectroscopy.
In dichloromethane solely dimeric species exist, whereas in benzene monomers are observed. Calculated electrostatic potentials
were used to rationalize the supramolecular structures of these two carbosilane compounds.