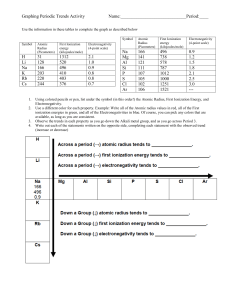
Study Guide: 1. Electron Configuration a. #52 Te: b. #85 At c. #118 2. Orbital Diagram a. #15 P b. #24 Cr c. #35 Br 3. Noble gas configuration a. #52 Te: b. #85 At: c. #118 4. Ionization energy a. Define ionization energy: b. Which of the following elements has the highest ionization energy? P, S, Cl c. Which of the following elements has the highest ionization energy? O, S, Se Study Guide: 5. Electronegativity a. Define Electronegativity: b. Which of the following elements has the highest electronegativity? P, S, Cl c. Which of the following elements has the highest electronegativity? O, S, Se 6. Atomic Radius a. Define Atomic radius: b. Which of the following elements has the highest atomic radius? P,S, Cl c. Which of the following elements has the highest atomic radius? O, S, Se 7. Calculate valence electrons a. How many valence electrons does Calcium have? b. How many valence electrons does CH3Cl have? 8. Draw Lewis dot structure a. Na b. Mg c. Al d. Ge e. S f. Cl g. Ar Study Guide: 9. Draw lewis structure a. PH3 b. CH3OH c. HCN 10. Determine if the lewis structure has resonance. If the structure has resonance draw the other resonance structures. a. b. 11. Determine where the double bond goes: 0-C-F 14. Define Paulii Exclusion principle: 15. Determine the charges of each of the following elements. a. Na b. Mg c. Al d. Si 16. Label the following families on the periodic table: a. Alkali metals b. Alkaline earth metals c. Noble gases d. Halogens e. transition metals e. P f. S g. Cl h. Ar Study Guide: 19. Define octet rule.