7.6 Solubility Equilibria
advertisement

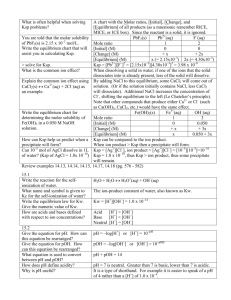
7.6 - Solubility Equilibria and Ksp Ksp represents the solubility product constant. It is not Kc or Keq. See p. 460. If you consider the dissolving of a slightly soluble compound in water you would write it as follows: CuCl (s) Cu+ (aq) + Cl- (aq) Recall that you do not include the solid in the equilibrium expression. Therefore the equilibrium expression is: Ksp = [Cu+ (aq) ] [Cl- (aq) ] Ksp = 1.7 x 10-7 at 25 oC (see appendix B4, page 725) This value indicates that copper(I) chloride is only slightly soluble in water. (very small Ksp) Watch out for compounds that are more complex like magnesium phosphate Mg3(PO4)2 Mg3(PO4)2 (s) 3 Mg2+ (aq) + 2 PO43- (aq) Ksp = [Mg2+(aq)]3 [PO43-(aq)]2 You can use the reaction quotient, Q, to calculate whether a system is in equilibrium or not. The reaction quotient can also be used to determine whether a precipitate will form. Q is the trial ion product. You are given the concentrations of the ions and then Q can be calculated. If Q < Ksp then no precipitate will form. The solution will be unsaturated. If Q = K sp then the solution is saturated. This will not form a precipitate. If Q > K sp then the solid will be present in the form of a precipitate eg) Will a precipitate form when 750.0 mL of Mg(NO3)2 (aq) at 4.00 x 10-3 mol/L is mixed with 300.0 mL of NH4OH(aq) at 2.00 x 10-2 mol/L? see Table 3, page 465 (solubility table) and Table 1 in Appendix B4 Common Ion Effect based off Le Chatelier's Principle adding a common ion to a saturated solution will shift the equilibrium to create more reactant (lower the solubility) lowering the solubility of an ionic compound by adding a common ion is called the common ion effect eg) Mg(OH)2(s) Mg2+ (aq) + 2OH- (aq) Add NaOH to the equilibrium system...and we expect the reaction to shift towards the reactants to oppose the change. An addition of a common ion in an equilibrium system MAY cause a precipitate to form IF the new Q value is greater than the Ksp We can use ICE charts and Ksp values to determine how much of an ionic compound will dissolve in a solution containing a common ion. See Tutorial 4, page 469.