Part III - Intermountain Healthcare
advertisement

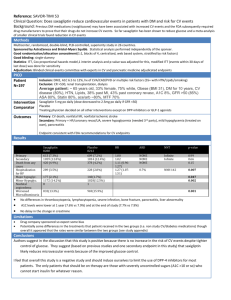
glucose. There may be some increased insulin sensitivity with its use also. Patients with Type II diabetes have on average dropped 7.7 kg and have had a decrease in hemoglobin A1c of anywhere from 0.7% to 0.85%. The main side effects include a slight increase in urinary tract infections (particularly those with a history of UTIs) and a rate of monilial infections in teenage and adult women of 7%. I did not know if this was a high rate but it turns out that in normal women the rate of monilial infection (yeast vaginitis) is 2%. The drug he was referring to is canagliflozin. I was fascinated with all of these drugs because in theory each one of them might produce improved control. Unfortunately, the improvement in control may not warrant the added expense and effort that these drugs would require. I am sure that there are going to be studies on these drugs over the next few years in pediatrics and I will look forward to seeing what they produce. With many of them there is a drop in weight which many of our teenagers would truly appreciate. Stay tuned because there will be more coming up. I was excited enough about these drugs that I went to the first part of a seminar on new developments with GLP-1 receptor agonists. I could only go to the first three talks because I had more pressing needs at other seminars going on simultaneously. Dr. Kathleen Dungan from Ohio State discussed differentiating current and emerging GLP-1 RA . She felt that in Type II diabetes these drugs are strong hemoglobin A1c lowering agents, they show little weight gain and little hypoglycemia. The main side effect is GI upset as I mentioned. Exenatide is given twice per day, liraglutide is given once per day and exenatide QW can be given weekly although it sounds as though it might be somewhat painful. She states in these patients the A1c drops by 1 to 1.8%. There is significant weight loss, greatest with exenatide and also with liraglutide. They all seem to be neutral in reduced gastric emptying. She also stated that they are in phase 3 testing on an oral preparation. Obviously this leads to better patient satisfaction. I wonder if this might be a drug that we could use experimentally with some of our patients. Dr. John Buse talked about GLP-1 receptor agonists in combination with basal insulin. The idea is that it increases portal insulin and decreases glucagon secretion. They are using a drug right now called IDegLira which is a combination of Degludec and liraglutide. They found when tested in Europe the A1c dropped 1.9% to 6.4%. Please remember they are using this drug with Type II diabetics who are supposed to have a lower hemoglobin A1c. They found that there was a modest weight loss. They also found there was a 32% less hypoglycemic rate compared to insulin alone and 50% less nausea than with liraglutide alone. This was the DUAL-1 study. The DUAL-2 study looked at IDegLira and compared it with Degludec alone. The hemoglobin A1c average dropped from 8.7% to 6.9%. He also reported that if you titrate the drug slowly that leads to less nausea. Dr. Paresh Dandona from my old stopping grounds in Buffalo talked about GLP-1 receptor agonist as adjunctive treatment in Type I diabetes. He again emphasized that they suppressed glucagon release, decrease appetite and diminish weight gain. His study was the first randomized study to look at a GLP receptor agonist in Type I. He used three doses of liraglutide, 0.6 mg, 1.2 mg and 1.8 mg. He found that the A1c with 0.6 mg dropped 0.4%, the 1.2 mg dose dropped the A1c by 0.8% and the 1.8 mg dose again dropped the A1c 0.4%. He found significantly better time to target numbers and much less time spent in the hyperglycemic range above 180 mg/dL. There was 1% more hypoglycemia with the use of the drug. He found that there was a decrease in the variability of blood glucose levels through the day. The basal insulin dose only dropped 0.8 units per day but the bolus insulin dropped 5 units per day. There was some weight loss and less carbohydrate intake of about 45 grams per day. The post prandial glucose was down significantly and the post prandial glucagon level was decreased significantly. 43 He also found reduced gastric emptying at the 1.2 and the 1.8 mg dosage. Interestingly he also found a decrease in blood pressure. Again, I can only introduce this subject to you today. I know Dr. Carol Foster and I have talked about our interest in using them and maybe someday we will able to do a controlled study to see what effect they have. Hopefully, the larger diabetes centers will do so before us and these drugs may be on the market for our patients in the future. Wouldn’t it be nice if the GLP-1 agonist was actually combined with the basal insulin so it did not require further injections? Only time will tell but it is encouraging. There were a few abstracts on the use of these drugs this year. A group from combined Tampa, Florida, Olm, Germany, Aurora, Colorado, Vienna, Austria and Indianapolis, Indiana (here you can see a wide variety of presenters) looked at the use of non-insulin medications for blood glucose control in Type I diabetes in the U.S. Type I Diabetes Exchange and the German/Austrian DPV registries. The analysis included 30,325 DVP patients and 16,893 patients from the Type I Exchange. They found non-insulin meds were rarely used in children less than 13 years of age (0.4% in the Type I Exchange and 0.2% in DPV). Metformin was the most commonly prescribed drug with higher use in Type I Exchange patients in the 13 to 26year-old category but was highest in use in DPV for adults greater than 50 years of age. They found that non-insulin med use increased with age, Type I diabetes duration and BMI Z-score (remember this is an obesity score). I have included their chart showing use according to age. You will see there is virtually no use of GLP-1 and SGLT-2 medications in the pediatric age group to this point. A group from Denmark presented work on 12 weeks of treatment with liraglutide as add on to insulin in normal weight but poorly controlled patients with Type I diabetes. They stated that this is the first randomized, double blinded (that means that the physician and patient does not know what medication they are taking), placebo controlled study using liraglutide 1.2 mg versus placebo in 40 patients with Type I diabetes. These patients had a hemoglobin A1c of 7.3% (their title makes me blush since they talked about poorly controlled patients and we oftentimes cannot achieve 7.3% A1c levels). They used it for twelve weeks and they found that the changes in hemoglobin A1c from baseline were similar with liraglutide and the placebo. The patients taking liraglutide had greater reductions in body weight and bolus insulin requirements compared with the placebo. Gastrointestinal side effects were significantly more frequent with liraglutide than with placebo. I have included their chart here. 44 They concluded “liraglutide added to insulin significantly reduces body weight and insulin requirements but has no additional effect on hemoglobin A1c in Type I diabetic patients inadequately controlled on insulin alone”. A group from Greenville, North Carolina did a meta analysis on the use of pramlintide as an add on therapy to insulin in Type I diabetes. As they pointed out “pramlintide is an amylin analog that reduces post prandial glucose thus improving hemoglobin A1c”. They used five studies total with 60 µg of pramlintide in two, 30 µg in two and 30 or 60 µg in one. They found that pramlintide was associated with a significantly greater hemoglobin A1c reduction and weight loss. There was also the expected greater reduction in total insulin use in the patients using pramlintide. Nausea, vomiting and reduced appetite were significantly higher with pramlintide. They found that severe hypoglycemic events were more common with pramlintide but did not reach statistical significance. I have included their chart also for your perusal. A group from Denmark reported on the LIRA-1 trial which studied the efficacy and safety of liraglutide added to insulin in Type I diabetes. There were 100 patients involved with a mean age of 47 years. The hemoglobin A1c at start was 7.3% (the Danes obviously do a much better job than we do). They found after 12 weeks that liraglutide reduced the hemoglobin A1c, body weight and daily insulin dose compared with placebo. However, by 26 weeks there was no difference in the hemoglobin A1c between the liraglutide group and the placebo group. The drop in body weight and daily insulin dose did remain significant, however. They found the 45 frequency of hypoglycemia was not different between the groups. Nausea occurred more frequently with liraglutide than with placebo (48% versus 7%). They concluded “liraglutide added to insulin treatment reduced body weight and daily insulin dose in overweight, poorly controlled patients with Type I diabetes but did not improve hemoglobin A1c compared with placebo at the end of treatment”. I have included their table also. That is about all I have to present to you on this topic right now. I would be surprised if I do not have more to present in the future. This may all turn out to be wishful thinking as so many of the approaches over the years tend to do. On the other hand, every once in a while one of these ideas takes off and it is incorporated in routine use. We can only see when the time comes. Hypoglycemia Next we come to a fairly sobering topic that has had a profound effect on our ability to control diabetes over the years. I want you to remember that much of what I am reporting deals with adults, many of whom have varying degrees of hypoglycemia unawareness. This is not a discussion of hypoglycemia in children. This symposium took place first thing Monday morning. This was our next to last day of meetings so already we were experiencing a good deal of fatigue. Dr. Brian Frier first presented hypoglycemic morbidity and costs in diabetes. He went through many of the acute problems with hypoglycemia including falls, fractures, dislocations and driving mishaps. He also discussed the greater incidence of myocardial infarctions and arrhythmias in terms of the cardiovascular effect. He reviewed also the acute effects on the brain leading to coma, seizures and cognitive (basically thinking) dysfunction. He pointed out that cognitive function deteriorates at about 54 mg/dL on average and that the recovery lags up to one 46 hour. Obviously mood changes are negative and when tested accuracy was preserved but the speed of cognitive function was diminished. The problems in driving include patients driving too fast or too slow, having inappropriate breaking and crashes. He said that there were 30 serious events per month amongst drivers with diabetes in the United Kingdom. There were three to five deaths per year. I find that hard to believe. I would have thought that number of deaths would have been significantly greater since we seem to have one death every five years or so in our group alone. He pointed out that the effects on the heart can be twofold. There can be an increase in the hemodynamics leading to a myocardial infarction in an already compromised heart. There also can be electrophysiologic events that can lead to sleep bradycardia (slowing down of the heart) and ectopic beats during sleep. He says that sleep bradycardia is eight times more common in diabetics and ectopic beats four times more common in diabetics. Interestingly, hypoglycemia can increase blood coagulation for up to one to two weeks and increase inflammation in the heart for up to two days. The patients with hypoglycemia unawareness have a six fold greater chance of severe hypoglycemia. Among long-term effects that he mentioned was that children with frequent hypoglycemia are vulnerable to IQ reduction. He did not know if hypoglycemia in adults led to a greater risk of dementia when old. He also reviewed the economic costs. Obviously there is the treatment cost including paramedics, ER visits and hospital admissions. There are the costs of medical follow-up and loss of productivity when someone misses work. Then there is, finally, the cost of co-morbidities. Interestingly, only 10% of episodes of severe hypoglycemia go to the hospital. In the United States there are 5 million visits in the emergency rooms each year for severe hypoglycemia and 25% are admitted. This number is much greater in the elderly population than in our population. The Type II diabetes costs are considerably more than the Type I but only because there are so many more Type II patients. He concluded by saying, “Please remember that hypoglycemia has a high burden and is an avoidable event.” Dr. Phil Cryer talked about hypoglycemia mortality in diabetes. He pointed out once again that hypoglycemia is the limiting factor in glycemic management of diabetes and precludes the maintenance of euglycemia (normal glucose levels) in our patients. He stated that deaths due to hypoglycemia mostly occur secondary to arrhythmias. He estimates that about 4 to 10% of all deaths in Type I diabetes is directly related to hypoglycemia. In the DCCT follow-up (remember this study from multiple years review) 8% of the patients died from hypoglycemia. He said that rarely are the deaths due to brain death. They usually are due to decreased baroreflex sensitivity and increased catecholamine release. Dr. Simon Fisher looked at arrhythmias and mortality associated with insulin induced hypoglycemia. He stated that mortality usually came about via inflammation/athrothrombotic events, ischemia or arrhythmias. He addressed the question of the “dead in bed syndrome”. He felt that the most common causes were either a fatal seizure, decreased respiration secondary to CNS dysfunction, brain death from neuroglycopenia, fatal arrhythmias and hypokalemia secondary to insulin use. He quoted a study that looked at the generation of arrhythmias in the presence of neuroglycopenia in animals. The first stage was a QTC wave expansion, then premature PVCs (premature ventricular contractions), then first and second degree heart block, then sinus bradycardia, then third degree heart block (which is atrial ventricular disconnection) and then rarely ventricular fibrillation. You really do not need to understand any of this; just to say that there seems to be a cascade of events that occur leading to cardiac arrhythmias. He did point that adrenergic blockade with both alfa and beta blockers prevents death due to hypoglycemia. The death is prevented because it prevents prolonged tachycardia. Interestingly, he felt that seizures did not lead to respiratory arrest or death. Dr. 47 Geremia Bolli from India discussed some of the common mistakes in using insulin. He stated that we need to keep in mind that we are talking both portal vein (the vein to the liver into which the pancreas releases insulin) insulin levels and peripheral levels. Portal vein levels are two to four times higher than peripheral levels so that when we give insulin we are developing peripheral hyperinsulinemia. He stated that we have very narrow therapodynamics. He stated that 50% of all medication errors in the United States are due to insulin. Half of these occur in patients 60 years or older. I thought they only occurred when I was on call. They lead to 9.2% of all emergency department admissions. He stated that one of the common problems is clinician errors. He felt that this was due to too ambitious glucose goals. He also pointed out that there were many self-administrative errors (and he included giving insulin into lypohypertrophy) and self-monitoring errors He stated that our patients do not consider the timing of insulin correctly in terms of food and exercise and that leads to many mistakes. He stated that there is a fair amount of using the wrong insulin but that pens have been helpful to prevent some of these issues (again, I think this only occurs when I am on call) and the other common error is miscalculating the ratio dose. He emphasized at the end of his talk that we need to individualize plasma glucose targets and not have universal targets for everyone. This was a fairly somber session. Again, we are not talking pediatric diabetes as much as geriatric diabetes but the mechanisms still apply. If we are ever to have diabetic control to the degree that we all desire, we will have to learn to minimize hypoglycemia so that parents and patients (and doctors) are willing to use an insulin most effectively. Many of the problems that Dr. Bell described are commonplace in our clinic and we need parents particularly to look at their own insulin use to try to overcome these errors as much as possible. There were a few interesting abstracts both posters and oral presentations on hypoglycemia. In one of the late breaking abstracts, a group from San Diego looked at the incidence of hypoglycemia overtreatment in the Share Real Life use Population. They were talking about overtreatment with food that results in rebound hyperglycemia of greater than 180 mg/dL. This was judged from a group of patients (1,177 users) using the Dexcom Share system. There were over 50 thousand hypoglycemic events recorded. On average, each person had 0.96 events per day overall with 0.4 events occurring during the nighttime. Thus events were more common during the day (59%) than during the night (41%). Eighteen percent of the events had resultant hyperglycemia within 60 minutes and 26% had hyperglycemia within 90 minute of the hypoglycemic event. I have included their results in the table. 48 A group from Atlanta, Georgia looked at the impact of hypoglycemia on the ambulance service covering approximately 90% of Atlanta’s city limits. Hypoglycemia caused 39% of all diabetes related calls (1,468 per year). They were more likely to occur between 9 a.m. and 7 p.m. than overnight. There was no pattern of increase by month or by day. The patients calling in for hypoglycemia tended to be older (60 years or older) and slightly more likely to be male. Patients with hypoglycemia were 2.7 times more likely to have multiple calls over the year than patients who called for other reasons. To my knowledge this does not occur too frequently in our patients. I do know that some call the paramedics but most of our patients handle hypoglycemia appropriately either by feeding their child or using glucagon when indicated. A group in Tochigi, Japan looked at hypoglycemic attacks in diabetic patients while driving an automobile. They passed out a questionnaire to 337 diabetic drivers but I am reporting only on the Type I’s which were 54 of the patients. Thirty-five point two percent of the group with Type I diabetes had experienced hypoglycemia while driving. Nine point three percent of this group had a traffic accident due to hypoglycemia. Eighty-eight point nine percent of this group of Type I diabetics had taken preventive measures against hypoglycemia such as carrying glucose while driving. Unfortunately, only 18.5% of the group tested their blood before driving. They also reported that only 35.2% of the patients with Type I diabetes had been given instructions regarding coping with hypoglycemia while driving. Every one of our patients hear about driving with diabetes when we sign their first driver’s license form and each subsequent DMV form each year. There is no excuse for them not to know how to handle hypoglycemia while driving. I am not naïve, however, and I realized that many of our patients do not test before driving as requested. This study merely shows why it is so necessary. Please take head and test before driving and always have something available in case you should drop low. A group from Croatia looked at changing the time of insulin glargine from bedtime to breakfast and determining the risk of hypoglycemia. They looked at 18 patients with Type I diabetes, all of whom had hemoglobin A1c levels greater than 7%. After 12 weeks of morning versus bedtime glargine the hemoglobin A1c had dropped from 8.02% to 7.4%. There had been no significant change in total daily dose and the average body weight remained the same. They also noted a significant reduction in nocturnal and daytime hypoglycemic episodes per month per person. They concluded “transition from bedtime to morning basal insulin administration in poorly regulated Type I diabetics not only improved glucoregulation, decreased glucose variability and reduced number of nocturnal and morning hypoglycemic episodes, but also induced favorable changes in lipid profile without effecting body weight”. They did not guess at the mechanism of improvement. We have switched to morning insulin with some of our teenagers where it was obvious that they were forgetting to take their Lantus when prescribed at bedtime. I normally choose to do it at dinner rather than breakfast but this study is an interesting idea and we may have to try it on some of our patients. Finally, I wanted to put in a report from Dundee, United Kingdom. They were looking at the exposure to acute intense exercise and its ability to restore effective counter regulation following recurrent hypoglycemia. You first need to understand that recurrent hypoglycemia oftentimes results in the suppression of normal counter regulatory hormonal and physiologic responses to later episodes of hypoglycemia. This effect makes patients at greater risk for subsequent severe hypoglycemia. This relationship has been known for several years. They were doing an interesting study to see if exercise would re-sensitize the hypothalamic glucose sensing neurons and thus prevent later hypoglycemia. This study was done in rats (I know, but many of our parents think that their children are in the same category) and they found that by doing significant exercise after the rats had had frequent hypoglycemia, they were able to 49 re-establish glucagon and epinephrine responses to hypoglycemia which in theory would prevent serious hypoglycemia. They conclude “these findings are consistent with the hypothesis that recurrent hypoglycemia desensitizes hypothalamic glucose sensing neurons to subsequent hypoglycemia in that introduction of a novel stimulus may re-sensitize them. High intensity exercise may represent a novel therapeutic intervention for people with impaired hypoglycemia awareness”. Just another reason for exercise. Obviously these studies have to be carried over to humans eventually but this would be a very reassuring result. We know that children who have frequent hypoglycemia are at much greater risk for severe hypoglycemia. If a bout of serious exercise could prevent that, it would be wonderful. There were also some interesting studies on hypoglycemia in dogs but I thought probably they were not germane to this presentation. Commercial Exhibits I put the summary of the commercial exhibits far to the end this year because there really were not many exciting developments. Probably the most important one was from Medtronic, the Mini-Med Connect. This will now enable their continuous glucose monitor to be transmitted to any phone device. Initially it will be an Apple phone but they are planning for it to be useable with any cell phone. It is very similar to the Dexcom Share in that parents can now follow their children’s CGM readings wherever they might be. Obviously this is particularly important to parents of toddlers at preschool and young children in early elementary school. Dexcom has been providing this for quite some time now and Medtronic has finally gotten back into the ball game. The device will cost $109 and can be used with the Enlite continuous glucose monitor. I went to the Lilly booth and talked about PEG lispro. As you remember, it is hepatic preferential and therefore in theory more physiologic than our other insulins. The polyethylene glycol is removed in the liver and it prevents the insulin from being excreted by the kidney. It is a once a day drug and has a half life of about 30 hours. The problem is that it causes more triglyceride deposition in hepatocytes increasing the ALT and AST, which are common liver function enzymes. They are now doing studies to determine the impact of these changes. They feel that PEG lispro is still very much in the pipeline but it is a question of how long it will be before it is submitted to and passed by the FDA. I also went to look at the Afrezza booth. This is the new human insulin inhalation powder that was released this spring. It is not indicated for pediatric use. It comes in both 4 unit and 8 unit cartridges. Thus a patient would need more than one inhalation oftentimes for a meal and correction. These are single use cartridges and the inhaler is good for 15 days. It is a unit for unit conversion with subcutaneous insulin so 4 units of Humalog would be equal to 4 units of Afrezza. There is faster absorption and it lasts anywhere from 30 to 150 minutes. Again it is not indicated for pediatric patients and I would very much 50 hold my horses until long-term studies about pulmonary function come out. Obviously they have satisfied the FDA but so many things come from long-term follow-up rather than from the time required by the FDA. Sanofi was introducing their Toujeo insulin which is a U300 variant of Lantus. It comes only in pen form so that patients do not get confused and forget that it is three times more potent than Lantus. It has the same ph so that it stings more than the analog insulins. If the needle is left in for five seconds there is less irritation. The advantage of Toujeo is that it seems to have a flatter curve than Lantus. I do not know why increasing the concentration produces that but it seems to. I have used it in a few of my older patients but have very little experience with it thus far. OmniPod is working on a new handheld controller that will be touch screen. It will be thinner and will have a battery that needs recharging only every seven days. It will be much quicker and will contain a better food library with 80 thousand different foods. They are working on CGM compatibility but there is nothing to announce yet. This new device will probably be out in the summer of next year. Dexcom reported that their sensors now last seven days and probably an additional two to three days longer if in stable conditions. The GEN6 device will be out probably in the next 18 months and will require only a single calibration per day. They are working on a device that will require no calibrations whatsoever. It will be calibrated in the factory and the use of self blood glucose monitoring will be unnecessary (except for verification). I did not catch when that would be conceivably available. Finally, I looked at the True Matrix glucose system which is sold from Walgreens. It is not as accurate as some of our other devices but is considerably cheaper. It uses the True Result strip that costs $39.95 for 50 strips. Obviously this is considerably cheaper than our other brands. As I said, the price that you pay is that the accuracy is less good. They now have a True Management software which will enable us to download the meter and print out results much as we do with the other meters. That was one of the major frustrations that we had earlier. Patients could not download and we could not download the meter so our patients were losing a good deal of valuable information. It would not be my first choice because of the accuracy. And that is all that I had from the commercial section this year. Oftentimes we have much more of interest but everything seemed to be fairly status quo in Boston. Epidemiology (Along with Others) I wanted to conclude with a few epidemiology presentations. First was a report from the same group that had talked about the comparison of Type I Diabetes Exchange and the German/Austrian DPV earlier. They were looking at 33,258 pediatric patients and their title was “Glycemic Control is Worse in the U.S. Compared with Germany/Austria”. They found several interesting results. First, patients in the Type I Diabetes Exchange performed less self 51 monitoring of blood glucose per day versus those in DVP. The children and adolescents had higher hemoglobin A1c levels and a lower percentage meeting the goals of less than 7.5% than those in DPV. The proportion of patients having one or more severe hypoglycemic events in the past year was similar between the two registries but patients in Type I Exchange had more DKA. Pump use differed by registry for all age groups but pump use was lower in the Type I Exchange for younger children less than 6 but higher in Type I Exchange patients aged 6 to less than 18. They concluded “pediatric patients of all ages in the DPV achieved better clinical outcomes compared with the Type I Diabetes Exchange, despite higher pump use among Type I Diabetes Exchange children aged 6 to less than 18”. Their results are in the following table. I do not want this to be a competitive sport but it is too bad that we do not do as well as the Europeans. I was particularly interested in the difference in the 12 to less than 18-year-old group’s hemoglobin A1c levels. I guess we could generalize about lifestyle and parental involvement but I do not think anyone quite understands why there is such a difference. A group from Rochester, Minnesota (the home of the Mayo Clinic) presented a poster on unexpected stability of Type I diabetes incidence in a U.S. cohort 1994 to 2010. They pointed out that recent studies show that the incidence of Type I diabetes is increasing worldwide by 2 to 5%. They pointed out that virtually all of the patients in their county go to Mayo for diabetes care so that they can keep good statistics on the development of new onset diabetes. They reported that there were 233 new cases with an age and gender adjusted annual incidence rate of 9.2 per 100,000 population. The average annual incidence rates are higher in males than females (10.5 versus 7.7 per 100,000) across all ages. Of most importance was there was no significant linear trend in Type I diabetes incidence, as incidence appeared to increase from 1994 to 2002 and decrease after. Unfortunately we cannot keep good records of new onset in Utah since not all new onset patients come to Primary. I do know that our number of new onset patients each year has increased (it was 326 last year for an all-time record). However, this number includes patients from Idaho, Nevada and Wyoming. We must also keep in mind that the population keeps growing so that the incidence rate may not be increasing as much as it appears. I will be very interested to see other studies from across the nation to see if they are seeing a slowdown also. It would be wonderful if that is the case because it had been reported in years past that the incidence of Type I diabetes in children and young adults was increasing at about 3.2% per year which is an incredible increase. A study from Timisora, Romania looked at the incidence of Type I diabetes in Romanian children aged 0 to 14 years. They noted that the incidence of children aged 0 to 14 years raised extremely significantly during the studied interval being more than double in 2013 (9.71 per 100,000 per year) than in 1996 (4.37 per 100,000 per year). This 52 is a mean annual increase of 7.2%. This trend was noticed for all the age subgroups. Their results are on the following graph. Apparently it is more dangerous to live in Romania than Rochester. Obviously sample sizes are small and we need more data before we can truly say that the frequency is still rising rapidly or is starting to slow down. Trevor Orchard’s group in Pittsburg reported on hemoglobin A1c and mortality in adults with Type I diabetes. I will not go through all the numbers but they concluded that their data “suggests that mortality may be comparable to that of the general population for those with the most recent hemoglobin A1c of less than 7%”. They recognized that they were quoting a small sample size but those results were encouraging. Please note, however, that they were referring to patients with a hemoglobin A1c of less than 7.0%. Those of you with much higher A1c’s are not in this category whatsoever and cannot take solace from their results. So that is all that I have to show for this year. Now there were other sessions that I chose not to report. I did not go into the presidential lecture or the Banting research lecture which were interesting but not germane to this handout. I also chose not to discuss dysglycemia (hyperglycemia in the ICU, steroid induced hyperglycemia and neonatal hyperglycemia) or the symposium on Type II diabetes in pediatrics. The last day there was a fascinating symposium where a patient, a researcher and a clinician talked about their 50 years of experience with diabetes. It was truly an eye opener. I came away from the meeting exhausted as you can imagine. I earned 30 hours of continuing medical education in four and a half days. I then visited a cousin in New Hampshire 53 and a friend in Maine which helped restore the senses. Next year the meetings will be in New Orleans so I am sure I will have a good deal to report from the French Quarters. Again, I hope that these handouts are useful to you. In reviewing what I have written, I realize that there are not a lot of take home changes this year but I do think that there is a great deal coming in the near future. I suspect it will be quite a ride. 54