Notes – Chem 11 HL Intro to Energetics
advertisement

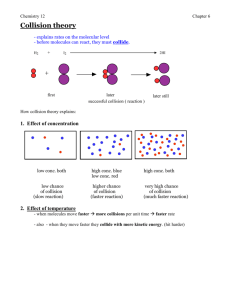
Chem 11 HL Unit 5: ENERGETICS AND THERMODYNAMICS Energy is defined as the ability to do work, i.e. move a force through a distance. It is measured in joules. Energy = force X distance (J) = (N X m) Thermodynamics is the study of energy and how it is interconverted. The first law of thermodynamics states that energy can be converted from one form to another and that the total amount of energy for a given system will remain constant. This law is often called the law of conservation of energy and states that energy can neither be created nor destroyed; it can only be converted between different forms. This means that for a given system we can account for and quantify all the energy changes. Temperature and Heat Heat, q, is a form of energy that is transferred from a warmer body to a cooler body, as a result of the temperature gradient. It is a measure of the total energy in a given amount of substance and therefore depends on the amount of substance present. Temperature is a measure of the ‘hotness’ of a substance. It represents the average kinetic energy of the substance, but is independent of the amount of substance present. At absolute zero, 0 K (-273.15°C), all motion of the particles theoretically stops and the entropy, S, of a system reaches its minimum possible value. Entropy (S) is a measure of the distribution of total available energy between the particles. The System and Surroundings In a chemical reaction, total energy is conserved. When examining the energy changes involved in a chemical reaction, we divide the universe into two parts: the system in which the chemical reaction is taking place, and its surroundings. 1 Chem 11 HL In an open system the transfer of matter and energy is possible across its boundary. For example, matter can be added to a beaker, and energy can be transferred through its sides. A closed system allows no transfer of matter, though energy may be transferred across the boundary. In an isolated system, matter can neither enter nor exit, but can only move around inside. Chemical Energy and Enthalpy When a chemical reaction takes place, the atoms of the reactants are rearranged to create new products. Chemical bonds in the reactants are broken and new chemical bonds are made to form the products. The internal energy stored in the reactants is known as its enthalpy, H. Enthalpy is an example of a state function. For a state function any change in value is independent of the pathway between the initial and final measurements. Energy is required to break the chemical bonds, i.e. bond breaking is an endothermic process. This energy is termed the bond dissociation energy. Energy is released when new chemical bonds are made, i.e. bond making is an exothermic process. Thermochemistry Thermochemistry is the study of heat changes that occur during chemical reactions. At constant pressure, the change in enthalpy ∆𝐻 is defined as the heat transferred by a closed system during a chemical reaction. Enthalpy change is usually denoted in kJ. Endothermic and Exothermic Reactions A chemical reaction in which heat is transferred from the system to the surroundings is defined as an exothermic reaction, with a negative ∆𝐻. Chemical reactions that absorb heat from their surroundings are defined as endothermic reactions, with a positive ∆𝐻. 2 Chem 11 HL Calorimetry The enthalpy change for a reaction can be measured experimentally by using a calorimeter. A calorimeter is any apparatus used to measure the amount of heat being exchanged with the surroundings. In a simple calorimeter all the heat evolved in an exothermic reaction is used to raise the temperature of a known mass of water. For endothermic reactions the heat transferred from the water to the reaction can be calculated by measuring the lowering of temperature of a known mass of water. To compensate for heat lost by the water in exothermic reactions to the surroundings as the reaction proceeds, a plot of temperature against time can be drawn. By extrapolating the graph, the temperature rise that would have taken place had the reaction been instantaneous can be calculated. Calculating Enthalpy Changes The heat involved in changing the temperature of any substance can be calculated from the following equation: Heat energy = mass (m) X specific heat capacity (c) X temperature change (∆𝑇) Or, q = mc∆𝑻 The specific heat capacity, c, of a pure substance is defined as the amount of heat needed to raise the temperature of 1 g of the substance by 1°C or 1 K. The specific heat capacity of water is 4.18 kJ kg-1 K-1; it requires 4.18 kilojoules of energy to raise the temperature of one kilogram of water by one kelvin. (sect. 2 of the Data booklet) 3 Chem 11 HL Example 1: When a 1.15g sample of anhydrous lithium chloride, LiCl was added to 25.0g of water in a coffee-cup calorimeter, a temperature rise of 3.80K was recorded. Calculate the enthalpy change of solution for 1 mol of lithium chloride. Example 2: 180.0 J of heat is transferred to a 100.0g sample of iron, resulting in a temperature rise from 22.0°C to 26.0°C. Calculate the specific heat capacity of iron. 4 Chem 11 HL Practice Problems: 1. 50.0cm3 of 1.00 mol dm-3 hydrochloric acid solution was added to 50.0cm3 of 1.00 mol dm-3 sodium hydroxide solution in a polystyrene beaker. The initial temperature of both solutions was 16.7°C. After stirring and accounting for heat loss, the highest temperature reached was 23.5°C. Calculate the enthalpy change for this reaction. 2. A student used a simple calorimeter to determine the enthalpy change for the combustion of ethanol. C2H5OH(l) + 3O2 (g) 2CO2 (g) + 3H2O(l) When 0.690g (0.015 mol) of ethanol was burned it produced a temperature rise of 13.2K in 250g of water. Calculate ∆𝐻 for the reaction. 3. 50.0cm3 of 0.200 mol dm-3 copper (II) sulphate solution was placed in a polystyrene cup. After two minutes, 1.20g of powdered zinc was added. The temperature was taken every 30 seconds and the following graph obtained. Calculate the enthalpy change for the reaction taking place. 5