thermo chemi first lesson
advertisement

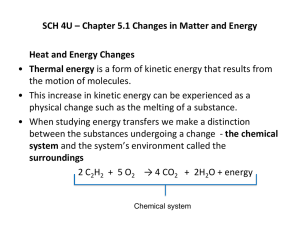
Thermochemistry (UNIT 2) Grade 12 Chemistry SCH4U0 What is THERMOCHEMISTRY? THERMOCHEMISTRY is the study of the energy changes that accompany physical or chemical changes in matter. Energy Transformations Heat vs. Temperature Heat (q) is the amount of energy transferred between substances (Units: Joules (J), kJ, kJ/mol) Temperature (T) is the measure of the average kinetic energy (energy of motion) of the particles in a sample of matter (Units: oC, oK, oF) Heat and Energy Changes What are the products when methane combusts in air? CH4 + 2O2 CO2 + 2H2O + energy Energy that is released from this CHEMICAL SYSTEM to the SURROUNDINGS is called thermal energy (heat). Since the molecules have greater kinetic energy, temp. of surroundings increases System and Surroundings The system is a well-defined part of the universe singled out for study. The surroundings is the remainder of the universe. In a closed system energy, but not matter, can be exchanged with the surroundings. In an open system , both energy and matter can flow into our out of the system. In an isolated system (ideal system) neither matter nor energy can move in or out. Exothermic and Endothermic Processes Heat (q) is a form of energy transfer. Units: 1 calorie (cal) = 4.184 J q>0 Heat is transferred from surrounding to system. Process is endothermic. q<0 Heat is transferred from system to surroundings. Process is exothermic. System q q Surroundings A basic calorimeter ) Surroundings everything else System with can as boundary Endothermic = absorbing energy Exothermic = releasing energy Law of conservation of energy = release and absorption of energy must be equal Heat and Temperature Change How is heat transferred related to the change in temperature of a system with mass m? q = specific heat (c) m T The specific heat (c) of a substance is the amount of heat required to raise the temperature of 1 gram by 1 oC. Al 0.90 J/gC H2O 4.18 J/gC Example Question 1 When 600 g of water in an electric kettle is heated from 20 C to 85 C to a make a cup of Rupinder’s favorite tea, how much heat flows into the water? Given: m = 600 g, ΔT = 85C – 20 C = 65 C c = 4.18 J/gC (from Table 1, p.301) Required: q (amount of heat transferred) Solution: q = mcΔT = (600)(4.18)(65) = 163 kJ Let's try this question now If 150.0 grams of iron at 95.0 °C, is placed in an insulated container containing 500.0 grams of water at 25.0 °C, and both are allowed to come to the same temperature, what will that temperature be? The specific heat of water is 4.18 J/g °C and the specific heat of iron is 0.444 J/g °C) Energy and Enthalpy Chemists give a special symbol, ΔH(delta H) to the heat change in a reaction This heat change is called ENTHAPLY ΔH (enthalpy) = energy absorbed or released to the surroundings when a system changes from reactants to products. ΔHsystem = - Qsurrounding HEAT HEAT HEAT HEAT Molar Enthalpy ΔHx: the enthalpy change associated with a physical, chemical or nuclear change involving 1 mol of a substance (x – is the letter used to indicate the type of change that is occurring) [Units: kJ/mol] ΔHvap ΔHsol ΔHfr ΔHcomb (See Table1 p.306) Types of Molar Enthalpies Example: COMBUSTION: (ΔHcomb ) CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) - 890kJ ΔHcomb = The amount of energy involved in a change depends on the quantity of matter undergoing that change. So, twice the mass of methane will release twice as much energy into the surroundings. Thus, ΔH = n ΔHcomb (ΔHcomb is obtained from a reference source) Enthalpies of Reaction H is an extensive property, so H depends on the amounts of reactants and products. What is H for the combustion of 11.0 g of CH4 in excess oxygen? CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) H = - 802 kJ 11.0 g CH4 mol CH 4 16 . 04 g CH 4 802 kJ 1 mol CH 4 = -550 kJ Example Question (p.309) In a calorimetry experiment, 7.46 g of KCl is dissolved in 100.0 mL of water at an initial T of 24.1 C. The final T of the solution is 20C. What is the molar enthalpy of solution of KCl? Calorimetry of Physical and Chemical Changes i) ii) iii) Three simplifying assumptions often used in calorimetry: No heat is transferred between calorimeter and the outside environment Any heat absorbed or released by the calorimeter material is negligible A dilute aqueous solution is assumed to have a density and specific heat equal to that of water Homework Questions p. 308 # 1 - 3 p. 310 # 4 p. 311 # 7, 9