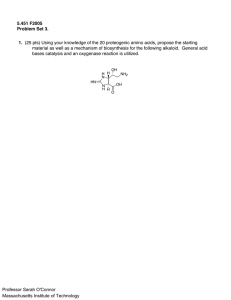
(10pts) Outline (structures) the biosynthesis of CTP from
advertisement

(10pts) Outline (structures) the biosynthesis of CTP from carbohydrate and simple (amino acids or one carbon unit) precursors. (16 pts) Thymidilate synthase (TS) and dihydrofolate reductase are important cancer targets. Why? Draw the mechanism of TS and explain (using structures and words) how fluorouracil inhibits this enzymes. (14 pts) Draw the biosynthesis of lysine. In one step, a one carbon fragment is lost. Draw the mechanism of this step. (You may start with cofactor-bound substrate and end with cofactorbound product.) (10 pts) Draw the mechanism of ribonucleotide reductase. What provides the reducing power for this reaction? How is this reducing power transferred to the enzyme? (i.e.What are the redox active molecules.) (12 Pts) Outline the biosynthesis of the following: Ornithine. Asparagine. Serine. (10pts) Describe three regulatory strategies used to control amino acid biosynthesis. (In general terms) (8pts) Describe the general mechanism used by cells to replace a carbonyl group with an amino group in nucleotide biosynthesis. Please use structures to illustrate your point. What are the nitrogen donors used in nucleotide biosynthesis? (6 pts) What are the carbon donors for synthesis of the purine base in nucleotide biosynthesis? (name(s) or structure(s)) (6 pts) What are the carbon donors for synthesis of the pyrimidine base in nucleotide biosynthesis? (Name(s) or structure(s)) EC (10pts) Pyrimidine biosynthesis contains a C-N bond-forming reaction that is unusual in biosynthesis. What is this reaction and why is it so unusual? What enzymic strategy is employed to make this reaction feasible?