ele12313-sup-0006-Population-Genetic-Model
advertisement

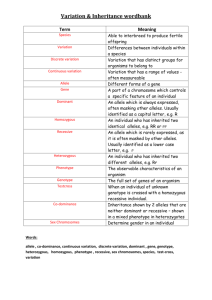
Detailed Methods for the Population Genetic Model We constructed a two-locus population genetic simulation model in which one locus influences a male ornamental trait, and the other a female preference. We assumed that individuals were diploid, the two loci were unlinked and autosomal, generations were non-overlapping, and population size was constant. As in previous models (e.g., Lande 1981), we assumed that males provide only gametes. 1. General setup of the model Suppose that the ornament under consideration is male color, and that the possible alleles at the ornament locus are T, B, and R, where T is the allele for the ancestral color (brown), B is a derived allele for blue coloration, and R is another derived allele for red coloration. For simplicity, assume there are only two alleles at the preference locus, b and r, where b encodes a preference for blue and r encodes a preference for red. The latter assumption is a simple way of implementing standing variation in female preferences. As noted in the main text, including an ancestral t allele (for no preference for T, B, or R) had only very minor effects on our results, though we include results below with a t allele in Cases 2 and 3 (Figs. S1, S2). Following our verbal descriptions above, our setup for all simulations was as follows. An ancestral population was assumed to have been divided into two geographically separate populations of equal sizes and equal genetic composition. At that time, we assumed that all individuals were homozygous TT at the ornament locus, the frequencies of r and b were each 0.5 (globally and within both populations), and the preference locus alleles were at Hardy-Weinberg equilibrium. Consistent with mutationorder divergence assumptions, in one population, the B allele arose at the ornament locus, whereas in the other population, the R allele arose at the ornament locus. We assumed an initial frequency of 0.001 for each derived ornament allele in the respective population in which it arose (and thus an initial frequency of 0.999 for the ancestral T allele). This was the starting point for all simulations. The two populations then evolved in allopatry for tA generations. Allele frequencies evolved during this period due to both selection and drift, explained in more detail below. Male ornaments were under direct selection due to the presence of the r and b alleles in the population. We considered a range of biologically realistic scenarios for how females with different preference genotypes would select among the possible types of males they encountered (below, Table S1). 2. Mechanics of allele frequency change over time With three alleles at the trait locus and two alleles at the preference locus, there were 18 possible distinct diploid genotypes (e.g., TTbb, TTbr, TBbr, etc.). Deriving analytic solutions for the changes of allele and genotype frequencies over time— especially given two sexes, linkage disequilibrium, and genetic drift—was not possible, so we used numeric simulations of populations over time as follows. Let fik,t be the frequency of the ith genotype in population k at time t. Assuming that genotype frequencies were the same within and between sexes, this means that a fraction fik,t of all females had the ith genotype. Assuming that males contributed only gametes and that there was no viability selection on genotypes, females of genotype fik,t thus produced a fraction fik,t of all offspring that formed generation t + 1. The genotypes of these offspring were determined from the frequencies of mating-pair types in which the female genotype participated. The frequency with which females of genotype i mated with males of genotype j depended upon the frequency of those males, fjk,t, weighted by the preference, pij, of a female of genotype i for males of type j. Assuming normal segregation and independent assortment, this information could then be used to determine the expected frequency of each genotype in the next generation. Because of linkage disequilibrium, genetic drift could not be modeled for each locus independently of the other; rather, it was necessary to model drift as affecting the frequencies of genotypes. Following results for the dynamics of a single locus (e.g., Graur & Li 2000), we assumed that the variance in genotype frequency in each generation due to drift could be approximated by i,t2 = fik,t(1 – fik,t)/(2Ne), where the effective population size, Ne, was held constant within a simulation and was varied from 103 to 106 by powers of 10 across different simulation runs. Hence, to account for drift, after reproduction but before the beginning of the next generation, the frequency of each (offspring) genotype was adjusted by an amount i,t drawn from a normal distribution with mean 0 and variance i,t2. After this adjustment, the frequency of any genotype that had dropped below 1/(2Ne) was set to zero. Note that the latter did not imply that the genotype would never arise again, unless that event also involved the global loss of one or more alleles that were part of the genotype. Genotype frequencies were scaled as needed to remain biologically realistic (i.e., between 0 and 1) and to sum to one to keep populations constant in size. 3. Migration and gene flow Migration was implemented by moving a fraction m of each genotype from one population to the other (i.e., migration was symmetric between populations and independent of genotype). Migration continued in this manner for tM generations, at which point simulations were halted. 4. Preference scenarios With 18 possible genotypes of females and males, there are 182 = 324 possible mating-pair types for which preference values, pij, must be specified. However, because a female's ornament alleles and a male's preference alleles are assumed to have no effects on a given mating interaction, specifying the pij simplifies to considering the interactions of each of the three possible female preference genotypes (--bb, --br, and -rr; dashes represent unspecified alleles at the other locus) with each of the six male ornament genotypes (TT--, TB--, TR--, BB--, BR--, and RR--). The list of six scenarios we considered and parameters for them are given in Table S1. 5. Extensions of the model to Cases 2 and 3 Case 2 assumes that males with derived traits (B and R) are more competitive than males with the ancestral trait (T). In extensions of the model to include this case, competition was assumed to change the relative frequencies of males that females encountered during the mating phase of the life cycle. B and R were assumed to be equally competitive with one another but more competitive than T. Hence, if Cij represented the relative competitive ability of a male with genotype ij at the trait locus, we assumed CBB = CBR = CRR = 1 + c > CBT = CRT = 1 + 0.5c > CTT = 1, where c was the competition coefficient (a fixed parameter for a given run; c = 0.1 in Fig. S1, below). To model Case 3, we adjusted the frequency of the genotypes of both sexes in the juvenile stage to represent viability selection for B and R over T. Let s (> 0) be the selection coefficient associated with the B and R alleles in this case. Viability selection was implemented by assuming that the relative survival rates to adulthood were SBB = SBR = SRR = 1 + s > SBT = SRT = 1 + 0.5s > STT = 1 References: Graur, D. & Li, W.-H. (2000). Fundamentals of Molecular Evolution. second edn. Sinauer Associates, Sunderland, MA. Lande, R. (1981). Models of speciation by sexual selection on polygenic traits. P. Natl. Acad. Sci. USA, 78, 3721-3725.