Density Lab
advertisement

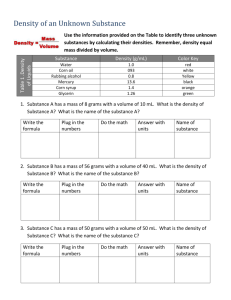
Name: ____________________________________________________ Date: ___________________ Introduction to Quantitative Measurement: Density Determination The study of chemistry involves not only observing changes in matter, but also measuring these changes. In fact, most chemical principles cannot be fully understood without obtaining and analyzing some quantitative data. The techniques of data analysis and measurement are important parts of chemistry. In this lab, density is used as a quantitative measure for comparing different substances. By carefully measuring the mass and volumes of substances, their densities can be calculated as follows: density mass volume or D = M/V In conducting this experiment, you will make several mass and volume measurements and use these measurements to determine the density of water, glycerin, and an unknown metal. You will then determine the precision of your results by calculating percent error. Careful attention should be given to the degree of uncertainty in your measurements. Use only those digits which are significant in your calculations in this experiment and in all of the other quantitative experiments that follow this year. The precision of your methods can be reported with your results in terms of percent of error. The percent error in calculations and measurements is a comparison of the differences between experimental results and accepted values, expressed as a percentage. Percent error can be determined as follows: % error = | experimental value – accepted value | x 100% accepted value Objectives: In this experiment, you will: 1. Determine the density of different substances from mass and volumes measurements. 2. Calculate the percent error in your results. Materials: 2 graduated cylinders (10 or 25 mL, and 50 mL) balance Water Glycerin Unknown Solid Samples Procedures and Methods: A. Determining the Density of Water: 1. Mass a dry 50 mL graduated cylinder to the nearest 0.01 g. Record in the data table. [Check with your teacher if you are in doubt about reading the balance.] 2. Fill the graduated cylinder with tap water roughly half way. 3. Re-mass the graduated cylinder. Record in the data table. 4. Carefully read the volume of the water in the graduated cylinder to the nearest 0.1 mL Read the volume at the bottom of the meniscus. Record in the data table. 5. You may leave the water in the graduated cylinder since you will use it for part C. B. Determining the Density of Glycerin: 1. Mass a dry 10 mL or 25 mL graduated cylinder. Record in the data table. 2. Fill the graduated cylinder to roughly the 9 ml mark with glycerin. Record exact volume in the data table, to the nearest 0.1 mL. 3. Re-mass the graduated cylinder. Record in the data table. 4. Return the glycerin to the container designated by your teacher. Clean the graduated cylinder thoroughly. C. Determining the Density of an Unknown Metal: 1. Obtain one sample of your lab group’s unknown from your teacher and measure its mass to the nearest 0.01 g. Record in the data table. 2. Use the 50 ml graduated cylinder with the tap water still in it. Record the initial volume to the nearest 0.1 mL in the data table. 3. Carefully immerse the solid in the water in the cylinder. Record the final volume to the nearest 0.1 mL. Record data in the table below. Data Table: A. Water B. Glycerin Mass of dry graduated cylinder = ________ g Mass of dry graduated cylinder = ________ g Mass of grad cylinder + water = ________ g Mass of grad cylinder + glycerin = ________ g Mass of water = ______ g Mass of glycerin = _____ g Volume of water = ______ mL Volume of glycerin = ______ mL C. Unknown Metal Sample # Mass of metal = ______ g Initial volume = ________ mL Final volume = ________ mL Volume of metal = ________ mL Calculations: 1. Use the data collected in parts A, B, and C to calculate the density for water, glycerin, and the unknown solid. The volume of the solid can be found by calculating the change in volume of water. Show work here: Water Glycerin Unknown Metal Sample 2. Calculate the percent error for each part. The teacher will list the actual densities of the unknowns following the lab. Show your work: Water Glycerin Unknown Metal Sample Data Analysis and Questions: 1. What were the major sources of error in your methodology (your procedure)? 2. The method used to find the density of a solid in part C, will not work for all solids. Why not? Suggest a method for determining the density of these solids. 3. Water is often used as a standard in making comparisons between substances. Based on your observations suggest a reason for using water as a standard.