Physics Formulas 2011
advertisement
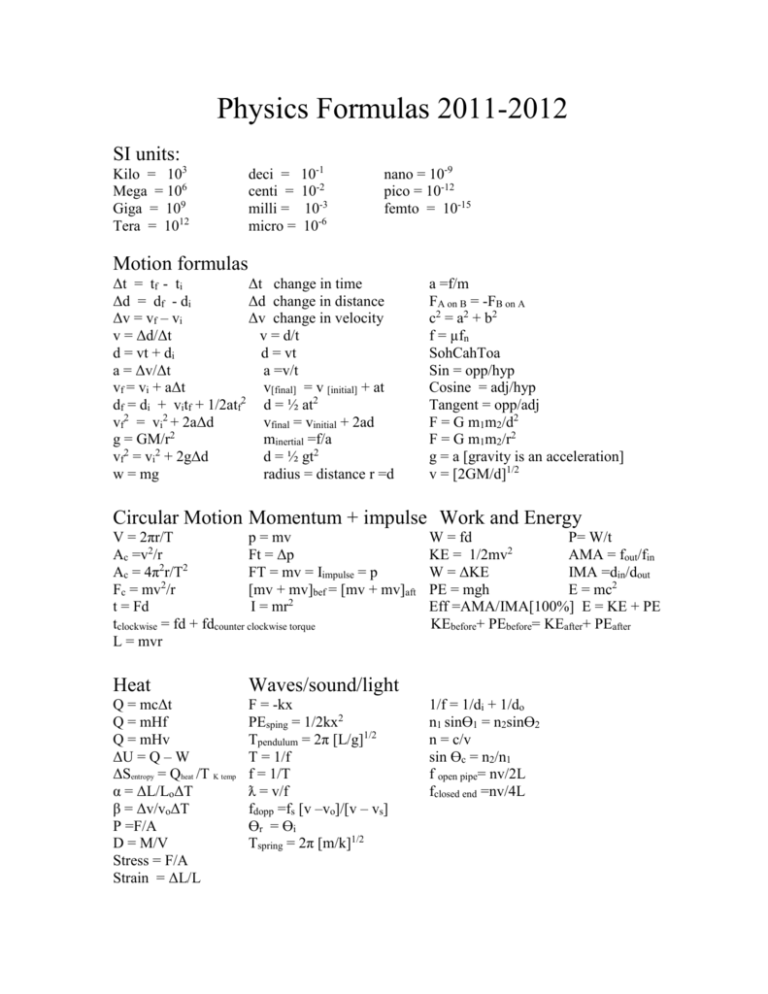
Physics Formulas 2011-2012 SI units: Kilo = 103 Mega = 106 Giga = 109 Tera = 1012 deci = centi = milli = micro = 10-1 10-2 10-3 10-6 nano = 10-9 pico = 10-12 femto = 10-15 Motion formulas Δt = tf - ti Δt change in time Δd = df - di Δd change in distance Δv = vf – vi Δv change in velocity v = Δd/Δt v = d/t d = vt + di d = vt a = Δv/Δt a =v/t vf = vi + aΔt v[final] = v [initial] + at df = di + vitf + 1/2atf2 d = ½ at2 vf2 = vi2 + 2aΔd vfinal = vinitial + 2ad g = GM/r2 minertial =f/a vf2 = vi2 + 2gΔd d = ½ gt2 w = mg radius = distance r =d a =f/m FA on B = -FB on A c2 = a2 + b2 f = µfn SohCahToa Sin = opp/hyp Cosine = adj/hyp Tangent = opp/adj F = G m1m2/d2 F = G m1m2/r2 g = a [gravity is an acceleration] v = [2GM/d]1/2 Circular Motion Momentum + impulse Work and Energy V = 2πr/T p = mv Ac =v2/r Ft = Δp 2 2 Ac = 4π r/T FT = mv = Iimpulse = p Fc = mv2/r [mv + mv]bef = [mv + mv]aft t = Fd I = mr2 tclockwise = fd + fdcounter clockwise torque L = mvr Heat Waves/sound/light Q = mcΔt Q = mHf Q = mHv ΔU = Q – W ΔSentropy = Qheat /T K temp α = ΔL/LoΔT β = Δv/voΔT P =F/A D = M/V Stress = F/A Strain = ΔL/L F = -kx PEsping = 1/2kx2 Tpendulum = 2π [L/g]1/2 T = 1/f f = 1/T ƛ = v/f fdopp =fs [v –vo]/[v – vs] Ɵr = Ɵi Tspring = 2π [m/k]1/2 W = fd P= W/t KE = 1/2mv2 AMA = fout/fin W = ΔKE IMA =din/dout PE = mgh E = mc2 Eff =AMA/IMA[100%] E = KE + PE KEbefore+ PEbefore= KEafter+ PEafter 1/f = 1/di + 1/do n1 sinƟ1 = n2sinƟ2 n = c/v sin Ɵc = n2/n1 f open pipe= nv/2L fclosed end =nv/4L Electricity and Magnetism F = kq1q2/d2 E = F/q V = W/q V = Ed C = q/v I = q/t P = IV V = IR P = I2R P = V2/R E = Pt P = W/t Rt = R1 + R2 + R3 . . . 1/Rt = 1/R1 +1/R2 +1/R3 . . . I = V/R F = ILB F = qvB V = nΔΦ/Δt EMF =Blv Ieff = .707 Imax Is/Ip=Vp/Vs=Np/Ns Veff = .707 Vmax ƛ =c/f Φ =AB Modern Physics E = hf E = hc/ƛ KE = hf - hfo P = hf/c = h/ƛ ƛ = h/p = h/ mv Ephoton = Ef - Ei E = mc2 Half-life = original [1/2]t hf = KE + W Constants Specific heat capacity (H20) 1 cal /gram°C [4187J/kg°C] (ice) ½ cal/g°C [2090 J/kg°C] Heat of fusion (H20) 80 cal/ gram [3.35 x 105 J/kg°C] Heat of vaporization (H20) 540 cal / gram [2.26 x 106 J/kg°C] 1 C = 6.25 x 1018 electrons 1 electron has charge of 1.6 x 10-19C 1 eV = 1.6 x 10-19 J Mass of electron = 9.11 x 10-31kg G = coulomb law 6.67 x 10-11Nm2/kg2 K =coulomb law = 9 x 109 Nm2/C2 Planck’s constant 6.63 x 10-31Js speed of light c [vac] 3 x 108 m/s 1 proton has charge of 1.6 x 10-19 C mass of neutron =1.67 x 10 -27kg mass of proton = 1.67 x 10-27kg speed of sound at STP 330 m/s Wavelength of Colors Violet light 400-440 nm Blue 440-480 nm Green 480-530 nm yellow light Orange red Units Meters Seconds meters/seconds meters/seconds2 kilograms newtons Joules coulomb farads volts hertz volts amps watt ohm 530-590 nm 590-630 nm 630-700 nm