Heat Transfer Experiments
advertisement

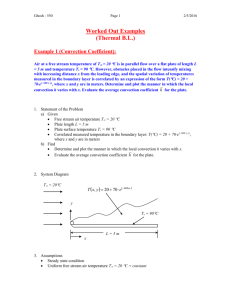
Heat Transfer To heat up or to cool down is always needed in a chemical plant Part of energy problem Source of energy: coal, natural gas, shale gas, nuclear reactor, solar, wind, hydraulic, geothermal etc. waste heat recovery transfer media: usually steam efficiency: can you estimate it for every transfer? 1 C: Heat Transfer Experiments Tubular heat exchanger: heat transfer coefficient h = f(Re); purpose - heating or cooling; existing in each factory; steam-electricity co-generation system汽電共 生; Drying: operation; sources of heating: conduction (less frequently), convection (hot air), radiation (lamp (wavelength), solar light, xenon lamp, etc) Picture taken from Wikipedia 2 3 Light spectrum for a xenon lamp (Google) 4 C1: Tubular Heat Exchanger Very traditional; steam is often used as the heating source; boiler to generate steam (may need licence to operate this equipment) Basic equation: q = h A T Overall heat transfer efficient (Uo or Ui): outside heat transfer coefficient ho, outside scale resistance Ro, tube heat transfer resistance kw, inside scale resistance Ri, inside heat transfer coefficient hi (scale resistance usually small) 1/Uo = 1/ho + Ro + xw/kw (Do/Dm) + Ri (Do/Di) + 1/hi (Do/Di); Dm = log mean dia. 5 liquid-side film heat transfer coefficient: ho, hi; usually assume other heat transfer resistance negligible, Q = m Cp (T out – T in) = hi Ai Tlm, with Tlm = (T2 - T1)/log (T2/T1) we may have co-current flow, counter-current flow, etc. In general: Nu = F(Re, Pr, L/D) Nu = Nusselt number = h D/k =convective heat transfer coefficient/conductive heat transfer coefficient Pr = Prandtl number = viscous diffusion rate/thermal diffusion rate = Cp/k 6 Logarithmic mean temperature difference Tlm 7 Many correlations proposed: e.g. Colburn j factor: Nu = 0.023 Re^0.8 Pr^1/3 (for Pr: 0.7 – 160) L/D < 60: entrance effect may not be neglected A simplified correlation: hi = a V^0.8 (1+ 0.0146 T) steam: saturated, superheating, super-cooling vent: safety purpose Source of heat: natural gas, diesel fuel (C8 – C21; BP: 200 – 320oC), coal, solar, waste heat, etc. heavy oil: very viscous, > C60, high percentage of aromatics, naphthalene, high amounts of NSO (chemical element); bottom product from distillation 8 B1 safety valve steam pressure steam inlet valve Ps B5 B4 B3 B2 hot water Tc steam trap Tc by pass valve cold water steam outlet Steam trap: to discharge condensate, noncondensable gases, with minimum loss of steam; usually automatic valve; 9 Different designs 10 C2: Drying Simultaneous heat and mass transfer Free moisture content, meaning some water may be chemically bond to solid material, may need “dehydration” moisture inside pores: may be slow to evaporate from air point view: percentage humidity (relative to saturation humidity) wet bulb temperature (adiabatic saturation temperature) vs dry bulb temperature vs dew point; humidity chart 11 Wet bulb globe temperature: originally developed by US marine corps. To determine heat stress of work WBGT = 0.7 Tw (wet bulb temp., humidity effect)+ 0.2 Tg (solar radiation) + 0.1 Td (dry bulb temp) 12 Humidity chart 13 Adiabatic saturation temperature – wet bulb temperature Air at T, H; water sprayed into to RH = 100%, system T = adiabatic saturation temperature cs (T-Ts) + Hs = Hs s; (H-Hs)/(T-Ts) = -cs/s adiabatic saturation line (on T-H diagram) Air at T, H flow over wet bulb to read T = wet bulb temperature hy (T-Tw) = Mb k w (Hw-H); (H-Hw)/(T-Tw)= hy/(Mb k w) psychrometric line cs hy/Mb k Lewis relation 14 pre-heat period, constant rate period, falling rate period (first & second falling rate); constant rate period reach “critical point”, then mass transfer becomes important 15 Taken from: manuals for best practice dryer 16 Dryer: with hot air drying + IR heater For industrial operation: usually in continuous mode (on a belt), tunnel kiln, etc. 17 Pictures taken from: Vandenbroek International website; drum dryer Spray drying; (others: fluidized drying, etc) 18 Double shell rotary drum dryer 19 In a typical phase diagram, the boundary between gas and liquid runs from the triple point to the critical point. Regular drying is the green arrow, while supercritical drying is the red arrow and freeze drying is the blue. (Wikipedia) 20