IT M , en
advertisement

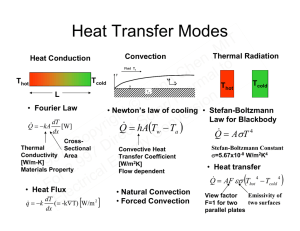
Importance of Heat T I M , n o e t h l C a g rm n e a h G /T ion © lar rs t e h o v g i S n r t o y c p C e o r C D i rg y e 7 7 n 9 9 9 9 E 2. r 2. cal i o r F ct e l E Courtesy of Lawrence Livermore National Laboratory. Used with permission. Vehicle Systems T I M , n o e t h l C a g losses 9kJMechanical m r n Exhaust a 10kJ 9kJ he n G T o Driving Gasoline / 6kJ 10kJ i r © s a r t 100 kJ l e Gasoline h 6kJ o v g Auxiliary S 30kJ n 35kJ 100kJri10kJ 30kJ t o y 35kJ c p C 10kJ ire o C DParasiticrgy e Coolant Exhaust 7 7 heat n losses 9 9 Parasitic 9 9 E Exhaust Coolant . . heat losses l 2 r 2 ca i o r t • FIn US,ctransportation uses ~26% of total energy. e l E Photo from Wikimedia Commons, http://commons.wikimedia.org Co-Generation in Residential Buildings T I M , In US, residential n and e o t h commercial buildings l C a consume ~35% energy g m r n e a supply h G /T ion © lar rs t e h o v g i S n r t o y c p C Entropy e o r Oil or i y C Thermal Power Heating g D Nat’l Gas r e 7 7 9 9 TE Recoveryn 9 9 E . . & & l 2 r 2 ca Electrical PowerRefrigeration Appliances Appliances i o r F ctPV Electricity e l E Photo by bunchofpants on Flickr. Image removed due to copyright restrictions. Please see any photo of the Honda freewatt Micro-CHP system, such as http://www.hondanews. com/thumbnails/2007/4/3/13644_preview.jpg Industrial Waste Heat T I Photos by arbyreed and toennesen on Flickr. 7 9 9 . 2 r 2. o F e l E M , n o e t h l C a g m r n e a h G /T ion © lar rs t e h o v g tS n i r o y c p ire C o C D rg y 7 ne 9 9 lE a c i r ct Fig. ES.1 in Hemrick, James G., et al. "Refractories for Industrial Processing: Opportunities for Improved Energy Efficiency." DOE-EERE Industrial Technologies Program, January 2005. Renewable Heat Sources T I M , n o e t h l C a g rm n e a h G /T ion © lar rs t e h o v g i S n r t o y c p C e o r C D i rg y e 7 7 n 9 9 9 9 E 2. r 2. cal i o r F ct e l E Photos by Jon Sullivan at http://pdphoto.org/ and NASA. Solar Thermal T I M , n o e t h l C a g rm n e a h G /T ion © lar rs t e h o v g i S n r t o y c p C e o r C D i rg y e 7 7 n 9 9 9 9 E 2. r 2. cal i o r F ct e l E Photos of solar hot water tubes removed due to copyright restrictions. Please see, for example, http://image.made-in-china.com/2f0j00KeoavBGJycbN/ Unpressurized-Solar-Water-Heater-VERIOUS-.jpg http://ns2.ugurpc.com/productsimages/solarevacuatedtube_202160.jpg http://www.treehugger.com/Solar-Thermal-Plant-photo.jpg Images by Sandia National Laboratories and NREL. http://media.photobucket.com/ Direct Energy Conversion T I M , n o e t h l C a g rm n e a h G /T ion © lar rs t e Thermoelectrics h o v g i S n r t o y c p C e o r C D i rg y e 7 7 n 9 9 9 9 E 2. r 2. cal i o r F ct e l Photovoltaics E COLD SIDE Image removed due to copyright restrictions. Please see http://web.archive.org/web/20071011185223 /www.eneco.com/images/science-new.jpg HOT SIDE http://www.solareis.anl.gov/images/photos/Nrel_flatPV15539.jpg Image by Nadine Y. Barclay, USAF. Thermophotovoltaics http://www.keelynet.com/tpvcell.jpg Courtesy of John Kassakian. Used with permission. Solar Spectrum T I M , 1600 AM1.5 Solar Spectrumn e o t h Energy Usable for Silicon PV Cells l 1400 C a g m r n 1200 e a h n G T o 1000 / i r © s a r t l e h o 800 v g i S n r t Bandgap of Silicon o y c p 600 C e (1.1 μm) o r i y C D rg 400 e 7 7 n 9 9 9 9 E . l 2. 200 2 a r 0 ric o F 0ct 0.5 1 1.5 2 2.5 3 e Wavelength (μm) l E Terrestrial Solar Spectrum (W/m2μm) 1800 Solar 1500 1000 500 0 0 0.5 1.0 1.5 2.0 2.5 3.0 Power (W/m2μm) (a) 1.5E4 1.0E4 0.5E4 0 0 2 4 6 (d) • • 8 10 12 Emissivity Absorptance Power (W/m2μm) Solar Thermophotovoltaics T I M , Insolation n o e t h l Optical ConcentratorC a g rm n e a Selective Wavelength (μm) h n G T o i Absorber r/ © s a r t Emitter l Irradiance e h o v From Emitterig S n r t Selective y TPV Cell o c p C e o r i y C g Wavelength (μm) D r e Thermal Management 7 7 n 9 9 Wavelength (μm) 9 9 E . . l 2Theoretical 2 maximum efficiency: 85.4%; comparable to that of infinite a r c i o number of multi-junction cells, but with only a single junction PV cell. r t F c Key Challenges: Selective surfaces absorbing solar radiation but ree lonly in a narrow spectrum near the bandgap of photovoltaic emitting E cells, working at high temperatures. 1.5 Absorber 1.0 0.5 (b) 0 0 1.5 5 10 (c) 15 Emitter 1.0 0.5 0 0 2 4 6 8 10 12 Solar Thermoelectrics T I 30.0 M , T =30 C n o e 25.0 h 700l Ct C a 600 C g m 20.0 n er 500 C a h 400 C n G T o 15.0 / i 250 C r © s a r t l ve 200 C h o g10.0 t S n i r 150 C o y c p ir5.0 e yC o C D rg e 7 7 0.0 n 9 9 0.5 1.0 1.5 2.0 2.5 9 9 E . . AVERAGE FIGURE OF MERIT ZT 2 r 2 cal Lowomaterialsricost and low capital cost, potentially high efficiency. t Develop materials with high thermoelectric figure of F Challenges: Key c e l merit; and E selective surfaces that absorb solar radiation but do not T -T hot cold EFFICIENCY (%) cold (b) • • re-radiative heat. 1st Law of Thermodynamics T I Closed: E2 − E1 = Q,12M− W12 Environment n o e t dE =Cδh Q − δW l a Q g W m r n dE e& & System a G /T=hQ −ioWn © ladtr rs t Boundary e h o State v g i S n r Process t Properties: o y c C Dependent Closed Systemop e Process r Open System C Di rgy Independent Quantities e 7 7 n 9 9 9 9 E 2. Er 2=. KEc+alPE + U (Internal Energy) + ... i o r t F Specific du c Heat C = [J/K - kg, or J/K - m ] e l E dT 3 2nd Law of Thermodynamics T I δQ M , S −S = ∫ + S (S ≥ 0)n T e to h l Entropy C a Entropy Entropy Change g m r n Transfer Generation State e a h G /T ion Properties r rsdS = 0 © Duringt a cycle:la e h ∫ o Heat Reservoir T v g i S n r t Q Q o y c 0 = − p C No rentropy generation e o Q T T C D i rg y e 7 7 Maximum Efficiency W Q −Q n 9 9 η= = 9 9 E (Carnot Efficiency) . . W Q Q 2 Qr 2 cal i o r C, T =23 C, η=40% F ct TT =223 =5800 K, T =300 K, η=95% e l Heat Reservoir T E 2 1 gen gen boundary h h h c h c h h c h c h o c c c h Tc = 1− Th o Thermal power plant η~40%, IC engines η~25% Microscopic Picture of Entropy T I M , n o • e t h • l C a g rm n e a h G /T ion 1 r rs P = © laProbability Boltzmann Principle t Ω e h o v g i S n r S = k B ln Ωy k =1.38x10 J/K ---Boltzmann constant t o c p C e o r i y C g D r • Constant Temperature • Constant Temperature e 7 7 n 9 9 and Closed Systems But Open Systems 9 9 E . . l 2 r 2 ca− E /( k T ) − ( E − μ ) /( k T ) Po( E ) =triAe P ( E ) = Ae F c e μ --- chemical potential (driving force for mass diffusion); l E • For Isolated Systems B B • Microstate: a quantum mechanically allowed state A total of Ω microstate Principle of equal probability: each microstate is equally possible to be observed -23 B average energy needed to move a particle in/out off a system Maxwell distribution T I ( ) ,M n o e t h l ⎡C m(v + va + v )⎤ P( v , v , v ) = A exp ⎢ ⎥ g m n ⎢⎣ er2k T ⎥⎦ a A box of gas h n G T o / molecules All Probability must normalize to one i r © s t ola er h v g ⎡ m(vt S i n r ⎛ m ⎞ + v + vo)⎤ y ⎟ A = ⎜⎜ A exp ⎢ec 1 = ∫ dv ∫ dv ∫ dv ⎥ p C ⎟ 2 π k T o 2 k T r ⎠ ⎝ C Di ⎢⎣ rgy ⎥⎦ e 7 7 ⎡ m(v + v + v )⎤ n ⎛ m ⎞ 9 9 Maxwell 9 9 ⎟⎟ exp ⎢ P ( v ,lvE, v ) = ⎜⎜ . . ⎥ Distribution 2 r 2 ca k T k T 2 π 2 ⎢⎣ ⎥ ⎝ ⎠ ⎦ i Fo ctr e l E 1 E = m v 2x + v 2y + v 2z 2 2 x x y 2 y 2 z z B ∞ ∞ x −∞ ∞ y −∞ 2 x 2 y 3/ 2 2 z z −∞ B B 3/ 2 x y 2 x 2 y z B B 2 z One molecule T I ⎡ m(v , +M v + v )⎤ 1 E = ∫ dv ∫ dv ∫ dv m(v + v + v )A exp ⎢ n ⎥ e o 2 2k tT ⎥⎦ ⎢⎣ h l C a g rm 3 n E = k T e a 2 h G /T ion © ∫ lar term s Equipartition Principle:tevery quardratic in microscopic r e h energy contributesig k T/2. So v n r t o y c p C J/K × 300K = 5.14 × 10 J kirTe= 1.38y× 10 How much o C g Is k T at room D r e ×10 J 5.14 7 7 temperature n 9 9 = 26 meV = 9 9 E 2. r 2. cal 1.6 ×10 J / eV i o r t F 3k T 3 ×1.38 ×10 J / K × 300 K c = 220 m/s v = = Oxygen Atom at 300 K e l m 16 ×1.67 ×10 kg E ∞ ∞ x −∞ ∞ y −∞ z 2 x 2 y 2 x 2 z −∞ 2 y 2 z B B E = B −23 -21 B - 21 B −19 −23 B − 27 Fermi-Dirac Distribution T I • From quantum mechanics M , • Energy levels are quantized n Energy : E = htνo = h ω e • Each quantum state can have h l maximum one electron C a Momentum : p = h/λ = hk g • Planck-Einstein Relation m r n h = h /(e2π ) a • Planck constant h=6.6x10 Js, G /Th ion r © with s • Consider one quantumt state an energy E at constant a r l e h temperature T. Thegstate cano have zero electron (n=0) or one v Saverage nnumber of electrons if ri iscthe electron (n=1). yWhat t o p observations? C one does many e o r C D i rg y ⎛ μ ⎞⎡ ⎛ E ⎞⎤ e 7 7 n ⎟⎟ ⎢1 + exp⎜⎜ − ⎟⎟⎥ 9 9 = A exp⎜⎜ 1 = ∑ Ae 9 9 E ⎝ k T ⎠⎣ ⎝ k T ⎠⎦ 2. r 2. cal i o r t of electrons in the state • Average F number c e l E -34 − ( E − μ ) /( k BT ) n = 0 ,1 B B Fermi-Dirac Distribution T I M • Average number of electrons in the state , n o e t h 1 l Fermi-Dirac = f = ∑ nAe C a Distribution ⎛ E −g μ⎞ m exp⎜⎜an ⎟⎟ + 1er G⎝ k T/T⎠h ion © lar rs t e h o v g At T=0K, μ is called i S n r t y ec Co Fermi level, E p o C Dir rgy F=1 for E<μ e 7 7 n 9 9 F=0 for E>μ 9 9 E . . 2 r 2 cal i o r F ct e l E − ( E − μ ) /( k BT ) n = 0 ,1 B FERMI-DIRAC DISTRIBUTION 1 0.8 f 1000 K 0.6 0.4 100 K 300 K 0.2 0 -0.1 -0.05 0 E-μ (eV) 0.05 0.1 Photons and Phonons T I M , Energy : E = hν = hω n e to h l : p = h/λ = h k Momentum C a g h = 6r.m n 6 ×10 Js; h = h /(2π ) e a h oofna quantum state: G /TEnergy i r © s t ola Ee=r⎛⎜ n + 1 ⎞⎟hω n = 0,1,2... h g t S nv ⎝ 2 ⎠ i r y ec Co p Zero point energy • Classical Oscillator o r C Di rgy Natural Frequency e 7 7 Spring n 9 9 9 9 E 1 K 2. r 2. cal ν = 2π M i o r F ctM 1⎞ ⎛ E = n + e ⎜ ⎟hω n = 0,1,2... Energy of Mode l 2⎠ ⎝ E • From quantum mechanics • EM waves are quantized, basic energy quanta is called a photon • Photon has momentum • Planck-Einstein Relation • Each quantum state of photon (an EM wave mode) can have only integral number of photons One Photon −34 Basic vibrational energy quanta hν is called a phonon Bose-Einstein Distribution BOSE-EINSTEIN DISTRIBUTION 5 T I M state • Consider one, quantum n o in thermal equilibrium e t h l − ( E − μ ) /( k T ) C a Pg( En ) =mAe n er a G /Th ion r rs Distribution © l•aBose-Einstein t e h o v g i S n r Average number of t o y c p C photons/phonons in one e o r C Di rgy mode (quantum state) e 7 7 1 n 9 9 f = 9 9 E . . l ⎛E−μ ⎞ 2 r 2 ca ⎟⎟ − 1 exp⎜⎜ i o ⎝ k T ⎠ F c tr e l Usually μ=0 E n 4 5000 K 3 1000 K 2 300 K 1 100 K 0 0 0.1 0.2 0.3 FREQUENCY (X10 14 0.4 0.5 Hz) B B Heat Transfer Modes T I M Thermal Radiation Convection , Heat Conduction n o e t h l C a T T T g T m r n e a L G /Th ion • Fourier Law © lawlaofr coolingrs• Stefan-Boltzmann • Newton’s t e h o Law for Blackbody dT v g & i S & =thA Q = −kA [W] r ( Q T −oTn ) dx y & = AσT c Q p C e Cross- o r y C Di Convective Stefan-Boltzmann Constant Thermal Sectional g Heat r Conductivity 7 σ=5.67x10 W/m K Area e Transfer Coefficient 7 n 9 9 [W/m-K] [W/m K] 9 9 E • Heat transfer . . Materials Property 2 r 2 cal Flow dependent ) Q& = AF εσ (T − T i o r • HeatF Flux ct • Natural Convection Emissivity of View factor e l dT • Forced Convection two surfaces F=1 for two & E Fluid Ta y cold hot uy ux x cold hot Tw w 4 a -8 2 4 2 4 hot q = −k dx [ (= -k∇T) W/m 2 ] parallel plates 4 cold Heat Conduction T I M Heat Conduction , n o e t h l C T T a g rm n L e a h G /T ion 1D, no heat generation © lar rs t e h o T T T T − − v g i S Q& = kA = n r t Ry L o c p C L e o r Thermal Resistance C DR i = kA rgy e 7 7 & Heat Current Q n 9 9 9 9 E 2. r 2. cal To T i r R F ct e l Convection E R=1 5 10 Diamond 4 10 cold hot Silicon 3 hot cold hot Thermal conductivity (W/mK) 10 cold th Copper Quartz single crystal (// to c-axis) 2 10 Stainless steel (type 304) 1 10 Ice th Fused quartz 0 10 Water (saturated) Helium (1 atm) 10 hot -1 cold th 10 th hA Air (1 atm) Steam (saturated) -2 10 1 2 10 Temperature (K) 3 10 Heat Conduction: Kinetic Picture T I M , n o e t Hot q h Cold l C a g rm n e a h G /T ion © lar rs vτ x t e h o v g S 1ri 1 n t o y − (nEv c) q = (nEv ) p C 2 ire 2 o y C g r d(Env ) vDτ dUedT q = -v τ97 =7− n 9 dx 3 dT dx dU J 9 9 E . . [ ] = C l 2v τ dT 2 m K a dT dT =− Cor = −kric 3F dx ct dx C = ρc e 1 Specific heat El x x x x x−vx τ x x+vx τ 2 x x • Energy per particle: E [J] • Number of particles per unit volume: n [1/m3] • Average random velocity of particles v x • Average time between collision of two particles τ---relaxation time • Average distance travelled between collision Λ=vτ---Mean free path • Volumetric specific heat x 3 2 Thermal Conductivity k = CvΛ 3 Density per unit mass Thermal Radiaton: Planck’s Law T I M Perfectly Basic Relations , Reflecting Wall n Frequency ν e o at T t h Angular Frequency ω=2πν l Inside the Cavity C a Wavelength λ EM Wave In g rm Wavevector magnitude k=2π/λ n Equilibrium at e a Wavevector k=(k ,k ,k Temperature T k h G /T ) ion ©c = νλlar ωrs= ck = c k + k + k t e h o ω(k): Dispersion relation (linear) v g i S n r ct o y energy in the cavity? p ire How much C o C D Ur=g2y hωf (ω,T ) = ∑∑∑ e λ 9λ7 λ 97 n L = 9,2 ,..., n ,... 9 E 2.2 2π 2r 2. 2 cal 2∫ (2πdk/ 2L ) ∫ (2πdk/ 2L ) ∫ (2πdk/ 2L )hωf (ω,T ) k =n o i r L 2 F ct dk dk dk e = 2 hωf (ω , T ) l ∫ ∫ ∫ (2π / L ) (2π / L ) (2π / L ) Two E polarization x y z 2 x ∞ x x x ∞ n x =1 n y =1 n z =1 x x ∞ ∞ ∞ ∞ y x x x x 0 x ∞ y 0 y x −∞ z 0 ∞ ∞ x −∞ z z y −∞ z 2 y 2 z Thermal Radiaton: Planck’s Law T I • Energy density per ω interval M ∫ ∫ ∫ hωf (ω , T )dk dk dk , n u (ω ) = hωf (ω ,e T )D(ω ) o 2V t h ( ) = f T k dk h , 4 ω ω π l hω C 1 a 8π ∫ = Planck’s law πg c ⎛ hωm ⎞ n exp⎜⎜ r ⎟⎟ − 1 2V ⎛ω ⎞ ⎛ω ⎞ e a = hωf (ω , T )4π ⎜ ⎟ d ⎜ ⎟ ⎝k T ⎠ n ∫ h G 8π ⎝c⎠ ⎝c⎠ T o / • Intensity: energy flux per unit i r © s ω U a angle r t olsolid = ∫ hωf (ω , T ) dω e h V π c g t S dAnv cu(ω ) hω i r 1 o y ( ) = = ω I c = ∫ hωf (ω , T )D(ω )dω p C e 4π 4π c ⎛ hω ⎞ o r ⎟⎟ − 1 i exp⎜⎜ y C D rg k T ⎝ ⎠ e 7 n Solid Angle Per unit wavelength interval = ∫ u (ω )d9 ω7 9 9 9 E . . l dA 1 I (ω )dω 4πch 2 r 2 ca dΩ = I (λ ) = = R i λ dλ ⎛ 2πhc ⎞ o D(ω)-density of states per r ⎜⎜ ⎟⎟ −1 exp t F whole space c unit volume per unit ⎝ k Tλ ⎠ e Planck’s law 4π angular frequency El interval 2V U= 3 8π ∞ ∞ ∞ x y z − ∞− ∞− ∞ ∞ 2 3 3 0 2 3 ∞ 2 B 3 0 ∞ 2 2 3 0 ∞ p 3 3 2 0 ∞ B 0 p 2 5 B Thermal Radiaton: Planck’s Law T I M Q& Wien’s displacement law , n o e t λ T = 2898 Kμmh l C a g rm n e a h n G T o / Emissive Power i r © s t ola er h g t S nv i Q& (λ ) = AπI (λ ) r o y c p C 1o hω e r =A i y 4π c ⎛C hω ⎞ g r ⎟⎟ − 1 D exp⎜⎜ e 7 ⎝ k T 9⎠ 7 n 9 9 9 E . . l 2 r 2 ca Total i o r Q& = ∫F Q& (λ )dλ = c AσtT e l E 2 EMISSIVE POWER (W/cm μm) max 3 2 2 B 10 4 10 3 10 2 10 1 5600 K 2800 K 1500 K 10 0 ∞ 800 K 4 0 10 -1 0 2 4 6 WAVELENGTH (μm) 8 10 MIT OpenCourseWare http://ocw.mit.edu 2.997 Direct Solar/Thermal to Electrical Energy Conversion Technologies Fall 2009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.