Electron Configuration Notes (Note Packet 2)
advertisement
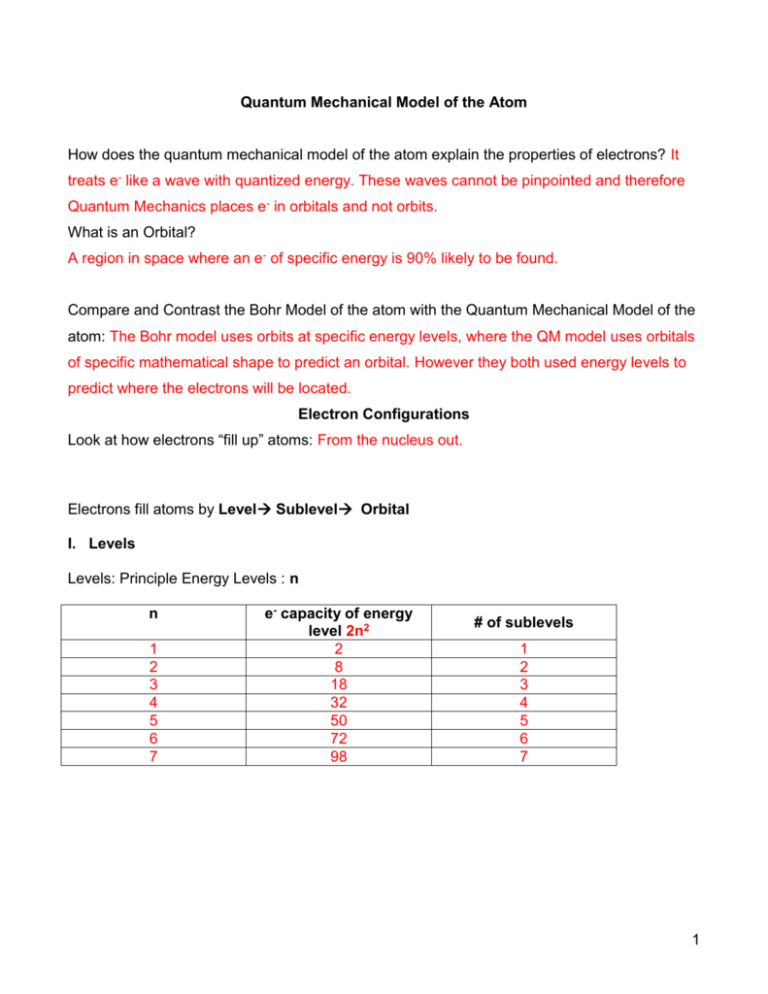
Quantum Mechanical Model of the Atom How does the quantum mechanical model of the atom explain the properties of electrons? It treats e- like a wave with quantized energy. These waves cannot be pinpointed and therefore Quantum Mechanics places e- in orbitals and not orbits. What is an Orbital? A region in space where an e- of specific energy is 90% likely to be found. Compare and Contrast the Bohr Model of the atom with the Quantum Mechanical Model of the atom: The Bohr model uses orbits at specific energy levels, where the QM model uses orbitals of specific mathematical shape to predict an orbital. However they both used energy levels to predict where the electrons will be located. Electron Configurations Look at how electrons “fill up” atoms: From the nucleus out. Electrons fill atoms by Level Sublevel Orbital I. Levels Levels: Principle Energy Levels : n n 1 2 3 4 5 6 7 e- capacity of energy level 2n2 2 8 18 32 50 72 98 # of sublevels 1 2 3 4 5 6 7 1 II. Sublevels Sublevel Names: s p d f g h i Sublevels are designated with the principle quantum # of the energy level, then the sublevel letter. n 1 2 3 4 5 6 7 Sublevel Names Electron Capacity 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 5g 6s 6p 6d 6f 6g 6h 7s 7p 7d 7f 7g 7h 7i 2 8 18 32 50 72 98 Sublevels denote the different energies electrons within a level can have. The type of sublevel the electron is in describes the shape of the probability distributions described by the quantum mechanical model for that electron. s sublevel: p sublevel: f sublevel: d sublevel: 2 III. Orbital Definition: A region in space where an e- of specific energy is 90% likely to be found. There can be no more than 2 e- per orbital. There are a given number of orbitals per sublevel: Sublevel s p d f g h i Number of Orbitals Orbital Designation 1 s 3 px py pz 5 dxy dyz dxz dx2-y2 dz2 7 Complicated math formulas 9 | 11 | 13 ↓ See pictures in text and draw p orbitals here: (page 109) See pictures in text and draw d orbitals here: 3 Fill up an atom with electrons filling Level Sublevel Orbital Orbital Level Sublevel 1 2 2 2 2 3 3 3 3 3 3 3 3 3 4 4 4 4 5 5 5 5 6 6 6 7 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 6s 6p 6d 7s 1s 2s 2px 2py 2pz 3s 3px 3py 3pz 3dxy 3dyz 3dxz 3dx2-y2 3dz2 4s(1) 4p(3) 4d(5) 4f(7) 5s(1) 5p(3) 5d(5) 5f(7) 6s(1) 6p(3) 6d(5) 7s(1) 2 Electrons per orbital 2 8 18 32 32 1s2 2s2 2px2 2py2 2pz2 3s2 3px2 3py2 3pz2 3d2xy 3d2yz 3d2xz 3d2x2-y2 3d2z2 4s2 4p6 4d10 4f14 5s2 5p6 5d10 5f14 6s2 18 6p6 6d10 2 7s2 2p6 3p6 3d10 4 Orbital Overlap The order that electrons fill up energy levels is not 1, 2, 3, 4, 5, etc. There is some overlap in sublevel energies. The periodic table can be used as a tool to read the order of sublevel energies n ns 1 1s1 1s2 2 2s1 2s2 3 3s1 3s2 4 4s1 4s2 3d1 3d2 3d3 3d5 3d5 3d6 3d7 3d8 3d10 5 5s1 5s2 4d1 4d2 4d4 4d5 4d5 4d7 4d8 4d10 6 6s1 6s2 5d1 5d2 5d3 5d4 5d5 5d6 5d7 7 7s1 7s2 6d1 6d2 6d3 6d4 6d5 6d6 6d7 2 3 4 5 6 7 7 1s2 np 2p1 2p2 2p3 2p4 2p5 2p6 3p1 3p2 3p3 3p4 3p5 3p6 3d10 4p1 4p2 4p3 4p4 4p5 4p6 4d10 4d10 5p1 5p2 5p3 5p4 5p5 5p6 5d9 5d10 5d10 6p1 6p2 6p3 6p4 6p5 6p6 6d9 6d10 6d10 7p1 7p2 7p3 7p4 7p5 7p6 (n-1)d n (n-2)f 6 4f 4f 4f 4f 4f 4f 4f 4f9 4f10 4f11 4f12 4f13 4f14 4f14 7 5f0 5f2 5f3 5f4 5f6 5f7 5f7 5f9 5f10 5f11 5f12 5f13 5f14 5f14 Write electron configurations for: H: 1s1 He: 1s2 Li: 1s22s1 Be: 1s22s2 B: 1s22s22p1 C: 1s22s22p2 N: 1s22s22p3 O: 1s22s22p4 F: 1s22s22p5 Ne: 1s22s22p6 5 Exceptions Exceptions to the AUFBAU filling order: (filling up in order of increasing energy) Cr, Mo, Cu, Ag, Au These elements have electron configurations that are exceptions due to the stability of the filled or half –filled d sublevel. Cr: 1s22s22p63s23p64s13d5 Mo: 1s22s22p63s23p64s23d104p65s14d5 Cu: 1s22s22p63s23p64s13d10 Ag: 1s22s22p63s23p64s23d104p65s14d10 Au: 1s22s22p63s23p64s23d104p65s24d105p66s14f145d10 Configurations of Ions: Anions: Gain e- so add p electrons to adjust for charge. Cations: Lose electrons so remove electrons to adjust for charge. For Representative elements, remove last electron to be added FIRST. For Transition elements, remove s electrons FIRST, then d until the charge has been accounted for. Examples: S2- Ca2+ N3- Cu- Fe3+ Pb2+ Pb4+ Abbreviated Notation: Back up to the previous noble gas and place that symbol in square brackets. Now add the rest of the electron configuration. Examples: I Au Xe [Kr]5s24d105p5 [Xe]6s14f145d10 [Kr]5s24d105p6 6 Electron Spin Electrons behave as though they are spinning. Spinning charges create: a magnetic field 1. If spinning clockwise: ↑ 2. If spinning counterclockwise: ↓ When 2 electrons have the same spins, their spins are called: parallel ↑ ↑ Magnetic fields add constructively (reinforce eachother) Paramagnetic: elements containing 1 or more unpaired e- . These elements will be drawn into an external magnetic field. Ferromagnetic: elements with extreme paramagnetism. Ex: Fe When 2 electrons have opposite spins, their spins are called: paired ↑ ↓ Spins cancel: Magnetic fields add destructively Diamagnetic: elements with all spins paired, magnetic field cancels. These elements will NOT be drawn into an external magnetic field. They may be unaffected, or they may actually be repelled by an external magnetic field. To determine if elements are paramagnetic or diamagnetic, use the following: Hund’s Rule: Electrons occupy equal energy orbitals so that a maximum number of unpaired electrons results. Give each orbital in a sublevel one electron before you give any orbital in that sublevel a second electron. The Pauli Exclusion Principle: Electrons cannot exist in the same orbital with the same spin. Therefore if an orbital is full it will have one up spin and one down spin electron. Draw orbital diagrams to determine if e- are paired or unpaired. Use a dash to represent each orbital, and place orbitals within the same sublevel together. Fill orbitals with electrons from lowest energy to highest applying Hund’s Rule and the Pauli Exclusion Principle. 7 Are the following paramagnetic or diamagnetic? Cr: Fe Zn: P: Ar: para para dia para dia Excited States So far, all configurations we have looked at are for elements and ions in the ground state, or lowest possible energy state. This means all possible orbitals are filled from lowest level to highest. In an excited state, an electron absorbs energy and jumps up to any higher level, sublevel that has empty space. Example: Ground State Configuration for Oxygen: 1s22s22p4 Some of the possible excited states of Oxygen: 1s22s02p6 1s22s12p5 8







