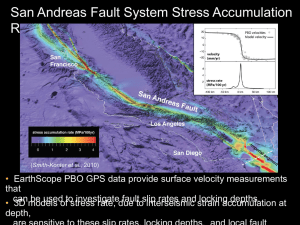
Phytoremediation of heavy metal contaminated soils by Abutilon
advertisement

Phytoremediation of heavy metal contaminated soils by Abutilon indicum V. Subhashini* and A. V. V. S. Swamy** *Faculty, Department of Environmental sciences, Acharya Nagarjuna University **Assistant professor, Department of Environmental sciences, Acharya Nagarjuna University Nagarjuna Nagar, Guntur, 522510. Andhra Pradesh, INDIA. Corresponding Author E- mail: arzavvsswamy1962@gmail.com ABSTRACT Heavy metals are the main group of inorganic contaminants and a considerable large area of land is contaminated with them due anthropogenic activities like fertilizers and emissions from municipal waste incinerators, car exhaust, residues from metalliferous mines and smelting industries, sludge or municipal compost and use of pesticides. The heavy metal toxicity causes serious threats to human and animal health due to their long term persistence in the environment. The technique using hyperaccumulator plants in phytoremediation is a cost-effective and new technology, to remediate the contaminated soil. In the present study a pot experiment was conducted using Abutilon indicum a shrub species for phytoremediation of Pb, Ni, Zn, Cd and Cr contaminated soils. Based on the BCF and TF the plant species recommended for lead, nickel and zinc Phytoextraction processes and cadmium and chromium Phytostabilization processes. Key words: Phytoremediation, Abutilon indicum, Bioconcentration Factor, Translocation Factor INTRODUCTION Worldwide, soil is being seriously degraded as a result of increasing industrial, agricultural and civil activities. The term heavy metal generally refers to a specific group of elements with metallic properties (metals and semimetals), often associated with contamination and potential toxicity or ecotoxicity [1]. The most common human-assisted routes for entry of inorganic contaminants heavy metal in particular, into agricultural and non-agricultural lands are via (1) disposal of industrial effluents, (2) sewage sludges, (3) deposition of air-borne industrial wastes, (4) military operations, (5) mining, (6) land-fill operations, (7) industrial solid waste disposal, and (8) use of agricultural chemicals such as pesticides, herbicides and fertilizers. The metal species commonly found in the soil as a result of the aforementioned human activities include copper (Cu), lead (Pb), zinc (Zn), nickel (Ni) , cobalt (Co), mercury (Hg) chromium (Cr) and cadmium (Cd) [2]. Metals of environmental concern are As, Cd, Cr, Cu, Pb, Hg, Ni, Se, Mo, Zn, Tl, Sb, and others [3]. Their anthropogenic application to soils is often related to the use of residuals, like biosolids, livestock manure and compost, adversely affecting human, crop and wildlife health [4]. Metal concentrations in soil typically range from less than one to as high as 100000 mg kg-1. The accumulation of metals/metalloids such as Arsenic (As), Cadmium (Cd), Chromium (Cr), Copper (Cu), Lead (Pb), Mercury (Hg), Nickel (Ni), Selenium (Se), and Zinc (Zn) through their mutagenic ability cause DNA damage and carcinogenic effects in the bodies of animals or human beings through food chain [5]. Heavy metals induce alterations of biochemical pathways in plants. In species which are sensitive to the metals cause inhibition of enzymes involved in photosynthetic reactions [6]. Heavy metals are the main group of 1 inorganic contaminants and a considerable large area of land is contaminated with them due anthropogenic activities like fertilizers and emissions from municipal waste incinerators, car exhaust, residues from metalliferous mines and smelting industries, sludge or municipal compost and use of pesticides [7, 8]. ` Phytoremediation is a novel, less expensive, efficient, environment and eco-friendly remediation strategy with good public acceptance [9, 10, 11]. Phytoremediation is the name given to a set of technologies that use different plants as a containment, destruction, or an extraction technique. This technology has been receiving attention lately as an innovative, costeffective alternative to the more established treatment methods used at hazardous waste sites [12, 13, 14, 15]. Phytoremediation, which uses plants to take up metals, is a cheap alternative technology, which is solar-driven and performed directly in situ [16]. Removing heavy metals through harvestable biomass is an efficient technique for inorganic pollutants. Plants used for this purpose should ideally combine high metal accumulation in shoots and high biomass production. Acacia nilotica bark serves as an adsorbent of toxic metals. Bark (1 g) when added to 100 ml of aqueous solution containing 10 mg ml-1 metal solution exhibited different metal adsorption values for different metals. The order of metal adsorption being Cr > Ni > Cu > Cd> As > Pb [17]. Jin-Hong et al. [18] in their study of twelve wetland species reported, Polygonum hydropiperoides Michx (smartweed) as the best for heavy metal phytoremediation, due to its faster growth and high plant density [19]. Sunflower (Helianthus annuus L.) and Indian mustard (Brassica juncea Czern.) are the most promising terrestrial candidates for metal removal in water. The roots of Indian mustard are effective in the removal of Cd, Cr, Cu, Ni, Pb, and Zn and sunflower removes Pb from hydroponic solutions [20]. MATERIALS AND METHODS A brief description of the plant species for the present study: Abutilon indicum, G. Abutilon indicum Plant and Roots 2 Abutilon indicum (Indian Abutilon, Indian mallow); is a small shrub, native to tropic and subtropical regions and sometimes cultivated as an ornamental plant [21]. This plant is often used as a medicinal plant and is considered invasive on certain tropical islands. In traditional medicine, A. indicum is used as a demulcent, aphrodisiac, laxative, diuretic, pulmonary and sedative (leaves) [22]. The bark is astringent and diuretic; laxative, expectorant and demulcent (seeds); laxative and tonic, anti-inflammatory and anthelminthic (plant); analgesic (fixed oil); diuretic and for leprosy (roots). The plant is very much used in Siddha medicines. The plant tends to have a weedy character, often found growing in disturbed sites [23]. Abutilon indicum seedlings were grown in pots filled with garden soil. The seedlings were collected from the uncontaminated soils. All the selected seedlings were of uniform size and free of any disease symptoms. The heavy metals selected for the study was lead, nickel, zinc, cadmium and chromium, the metal uptake was estimated in root, stem and leaves for every 20 days for a total period of 60 days. In addition a control set of experimental pots was also maintained. The heavy metal solutions of 5mg/L was prepared from the stock and administered to the plants and care was taken to avoid leaching of water from the pots. The metal uptake was estimated once in every 20 days up to 60 days (2 months). The sample plants were removed from the pots and washed under tap water and then with distilled water. The collected plants were air dried, then placed in a dehydrator for 2-3 days and then oven dried for four hours at 100 ºc. The dried samples of the plant were powdered and stored in polyethylene bags. The powdered samples were subjected to acid digestion. 1gm of the powdered plant material were weighed in separate digestion flasks and digested with HNO3 and HCl in the ratio of 3:1. The digestion on hot plate at 110ºc for 3-4 hours or continued till a clean solution was obtained. After filtering the filtrate was analyzed for the metal contents in AAS. The metal concentration, transfer and accumulation of metals from soil to roots and shoots was evaluated in terms of Biological Concentration factor (BCF) or Bioconcentration Factor (BCF) and Translocation Factor (TF). The TF value increases with increasing ability of the plant to translocate metals to stem and leaves. Thus the plants showing high BCF and TF values (greater than one) are suitable for phytoextraction. While the plants showing TF value less than one can be used for phytostabilization. Bioconcentration Factor (BCF): Metal concentrations in plants vary with plant species. The concentration, transfer and accumulation of metals from soil to roots and shoots was evaluated in terms of Biological Concentration Factor (BCF), Translocation Factor (TF). Biological Concentration Factor (BCF) was calculated as metal concentration ratio of plant roots to soil [24]. The Bioconcentration Factor (BCF) of metals was used to determine the quantity of heavy metal absorbed by the plant from the soil. This is an index of the ability of the plant to accumulate a particular metal with respect to its concentration in the soil [25]. Translocation Factor (TF): Translocation Factor (TF) was described as ratio of heavy metals in plant shoot to that in plant root [26, 27]. To evaluate the potential of this species for Phytoextraction, the Translocation Factor (TF) was calculated. This ratio is an indication of the ability of the plant to translocate metals from the roots to the aerial parts of the plant. Metals that are accumulated by 3 plants and largely stored in the roots of plants are indicated by TF values <1, with values greater indicating translocation to the aerial part of the plant [24]. RESULTS AND DISCUSSION: Accumulation of Lead (mg/kg) in Abutilon indicum: The plant parts were analyzed to estimate the accumulation of lead by 20th, 40th and 60th days. The accumulation of lead was lowest in the roots and highest in the stem. The lead that is absorbed from the soil by the roots is translocated to the above ground stem and leaves. The translocation of lead from roots to stem was higher compared to that of the stem to leaves. Most of the lead that is translocated to stem remained in the stem and gradually accumulated to the tune of 17.21 mg/kg. The accumulation was consistent throughout in the root system. The accumulation of lead showed a sudden increase by 20th day in the stem from 16.81 to 31.24 mg/kg and from then the accumulation was though marginal, it was consistent. Lead accumulation in the leaves also showed similar trend but with less concentration. Table 1: Accumulation of Lead (mg/kg biomass) in different plant parts of Abutilon indicum during the experimental period. Plant part Control Leaf 5.69±0.32 Stem 16.81±0.13 Root 28.78±0.08 Total Accumulation 51.28 20th day 40th day 12.84±0.08 15.73±0.12 31.24±0.19 32.56±0.19 29.63±0.17 29.82±0.1 73.72 78.11 Total 60th day Accumulation 17.76±0.16 12.07 34.02±0.08 17.21 30.03±0.05 1.25 81.81 30.53 Concentration (mg/kg) LEAF 100 STEM 80 ROOT 60 TOTAL ACCUMULATION 40 20 0 Control 20th day 40th day 60th day Total accumulation Experimental days Fig 1: Accumulation of Lead in Abutilon indicum during the experimental period Accumulation of Nickel (mg/kg) in Abutilon indicum: The total accumulation of nickel in the roots was lowest among the plant parts (6.9 mg/kg) during the whole experimental period. Out of 4 the total nickel accumulated by 60th day (30.44 mg/kg) only 6.90 mg/kg is accumulated in the roots while the accumulation in leaves and stem was more or less equal (11.57 and 11.97 mg/kg, respectively). The results reveal that the nickel that is absorbed from the soil is translocated to roots and in turn the roots have translocated to the stem and then to leaves. The leaves and stem of the Abutilon indicum have the same potential to absorb nickel. Table 2: Accumulation of Nickel (mg/kg biomass) in different plant parts of Abutilon indicum during the experimental period. Plant part Leaf Stem Root Total accumulation Control 1.28±0.50 2.6±0.13 9.7±0.08 20th day 5.08±0.08 7.07±0.16 9.88±0.07 40th day 10.85±0.13 11.71±0.18 10.51±0.06 60th day 12.85±0.19 14.57±0.05 16.6±0.03 Total Accumulation 11.57 11.97 6.9 13.59 22.03 33.08 44.02 30.44 Concentration (mg/kg) LEAF 50 STEM ROOT 40 TOTAL ACCUMULATION 30 20 10 0 Control 20th Day 40th Day 60th Day Total accumulation Experimental days Fig 2: Accumulation of Nickel (mg/kg) in Abutilon indicum during the experimental period Accumulation of Zinc (mg/kg) in Abutilon indicum: Abutilon indicum showed a wide variation in the accumulation of zinc in its parts viz. root, stem and leaves. Zinc accumulation was highest in leaves followed by stem and roots. The concentration of zinc increased rapidly from 40th to 60th day in all the plant parts and this revealed that the maximum accumulation took place during this period. The quantity of accumulation was also high compared to other metals in Abutilon indicum. Of the 278.08 mg/kg zinc accumulated in abutilon, approximately 50% was accumulated in leaves (140.82 mg/kg). 5 Table 3: Accumulation of Zinc (mg/kg biomass) in different plant parts of Abutilon indicum during the experimental period. Plant Part Control 20th day 40th day 60th day Total Accumulation Leaf 22.85±0.19 67.42±0.08 74.64±0.13 163.67±0.19 140.82 Stem 28.82±0.13 37.13±0.19 102.53±0.06 73.71 89.21±0.08 63.55 355.42 278.08 45±0.16 Root 25.66±0.08 76.81±0.06 83.63±0.09 Total Accumulation 77.32 181.37 203.28 LEAF Concentration(mg/kg) 400 STEM ROOT 300 TOTAL ACCUMULATION 200 100 0 Control 20th Day 40th Day 60th Day Total accumulaton Experimental days Fig 3: Accumulation of Zinc (mg/kg) in Abutilon indicum during the experimental period Accumulation of Cadmium (mg/kg) in Abutilon indicum: Cadmium metal is not an essential element for the plant. But it enters in to the plant body along with other nutrients through the root system. The highest concentration of cadmium was recorded in the roots by 60 th day showing a total accumulation of 14.08 mg/kg (55.6% of the total accumulation). Further it was observed that the maximum accumulation in roots took place between 40th and 60th day. From the beginning of the experiment, there was a consistent increase of the concentration of cadmium in leaves and stem. However, the total accumulation of cadmium in leaves and stem was much lower than in roots. The cadmium translocation was very poor from roots to stem and leaves in Abutilon indicum. 6 Table 4: Accumulation of Cadmium (mg/kg biomass) in different plant parts of Abutilon indicum during the experimental period. Plant Part Leaf Stem Root Total Accumulation Control 0.21±0.09 0.47±0.13 1.44±0.08 20th day 1.04±0.08 3.49±0.16 3.81±0.07 2.12 8.34 40th day 60th day 2.54±0.13 5.96±0.19 3.49±0.15 5.96±0.08 3.87±0.06 15.52±0.04 9.9 Total Accumulation 5.75 5.49 14.08 27.44 25.31 LEAF Concentration(mg/kg) 30 STEM 25 ROOT 20 TOTAL ACCUMULATION 15 10 5 0 Control 20th Day 40th Day 60th Day Total accumulation Experimental days Fig 4: Accumulation of Cadmium (mg/kg) in Abutilon indicum during the experimental period Accumulation of Chromium (mg/kg) in Abutilon indicum: Abutilon indicum showed a tendency of high absorption of chromium by the root system. The roots have accumulated 33.18 mg/kg (63.7%) of chromium during the experimental period and the accumulation in leaves and stem was low and consistent from 20th day up to 60th day. However, chromium was actively absorbed by the stem and leaves during the first 20 days of the experimental period. The chromium was not translocated to above ground plant parts and hence only 36% of the chromium was found in leaves and stem. 7 Table 5: Accumulation of Chromium (mg/kg biomass) in different plant parts of Abutilon indicum during the experimental period. Total Control 20th day 40th day 60th day accumulation 3.75±0.19 11.32±0.08 12.26±0.13 12.55±0.19 8.8 7.87±0.13 17.79±0.16 17.82±0.19 17.95±0.08 10.09 11.78±0.08 12.57±0.04 27.76±0.07 44.96±0.03 33.18 Plant part Leaf Stem Root Total accumulation 23.4 41.68 57.84 75.46 52.07 LEAF Concentration (mg/kg) 80 STEM ROOT 60 TOTAL ACCUMULATION 40 20 0 Control 20th day 40th day 60th day Total Accumulation Experimental period Fig 5: Accumulation of Chromium in Abutilon indicum during the experimental period Abutilon indicum: The quantities of metals absorbed by Abutilon indicum are moderate with respect to different parts and total accumulations. Cadmium and chromium were accumulated in substantially high quantities in roots (14.07 mg/kg, 33.18 mg/kg respectively). This revealed that the cadmium and chromium remained in the roots with being translocated to the above ground plant parts. A completely inverse picture was witnessed in case of zinc, nickel and lead. Highest accumulation of zinc was observed followed by lead in stem. In cases of lead, nickel, cadmium and chromium the total accumulation was more or less uniform in leaves and stem where as the leaves of Abutilon indicum showed greatest absorption of zinc. Moreover, no other metal except zinc showed a tendency to accumulate in such a (140.83 mg/kg) high concentrations in leaves. In other words, only zinc was translocated by roots very effectively to leaves when compared to other metals. No symptoms of toxicity were observed in Abutilon indicum even under such high concentrations. Zinc accumulation was very high in all the plant parts viz. leaves, stem and roots. The concentrations were nearly five times higher than other metals. Cadmium concentrations were lowest (5.75mg/kg in leaves and 5.49 mg/kg in stem). These differences of accumulations are attributed to the plant requirement. Zinc is required for plant for strength and to withstand environmental stress. Whereas cadmium and chromium enter the plant body along with the nutrients though they do not have any defined metabolic activity. Bio Concentration Factor (BCF) and Translocation Factor (TF) were also calculated. Lead BCF was 3.22 and TF was 23.44. Nickel BCF was 3.61 and TF was 3.41. Zinc BCF was 21.25 and TF was 3.37. Cadmium BCF was 69.52 and TF was 0.79. Chromium BCF was 3.83 and TF was 8 0.57. Based on the BCF and TF the plant species was good accumulator of zinc and chromium and moderate accumulator of Pb, Ni and Cd. Finally the plant species recommended for lead, nickel and zinc phytoextraction processes and cadmium and chromium Phytostabilization processes. CONCLUSION The plants suited for phytoremediation are fast growing and is able to produce a large amount of biomass. Phytoremediation is a developing technology which uses plants and their associated microbes for the remediation of soil contamination. This process is cost effective without creating disturbance to the soil. Based on the BCF and TF the plant species was recommended for lead, nickel and zinc phytoextraction processes and cadmium and chromium Phytostabilization processes. REFERENCES 1. Duffus JH. (2002). “Heavy metals” –A meaningless term? Pure Appl. Chem. 74: 793807 2. Saxena PK, KrishnaRaj S, Dan T, Perras MR, Vettakkorumakankav NN. (1999). “Phytoremediation of Heavy Metal Contaminated and Polluted Soils”. M. N. V. Prasad et al., Heavy Metal Stress in Plants. © Springer-Verlag Berlin Heidelberg 1999. 3. Basta NT, Ryan JA, Chaney RL. (2005). “Trace element chemistry in residual -treated soil. Key concepts and metal bioavailability”. J Environ. Qual. 34: 49 -63 4. Adriano DC. (2001). “Trace Elements in Terrestrial Environments. Biogeochemistry, Bioavailability and Risks of Metals”. Springer-Verlag, New York. 5. Knasmuller S, Gottmann E, Steinkellner H, Fomin A, Pickl C, Paschke A, God R, Kundi M. (1998). “Detection of genotoxic effects of heavy metal contaminated soils with plant bioassay”. Mutation Research, 420(1-3): 37–48 6. Bhardwaj R, MascareNas C. (1989). “Cadmium-induced inhibition of photosynthesis in vivo during development of chloroplast in Triticum aestivum.L”. Plant Physiol.Chem. 16: 40-48 7. Garbisu C, Alkorta I. (2003). “Basic concepts on heavy metal soil bioremediation”. The European Journal of Mineral Processing and Environmental Protection, 3(1): 58–66 8. Halim M, Conte P, Piccolo A. (2003). “Potential availability of heavy metals to phytoextraction from contaminated soils induced by exogenous humic substances”. Chemosphere. 52(1): 265–75 9. Singh A, Eapen S, Fulekar M. H. (2009). Potential of Medicago sativa for uptake of cadmium from contaminated environment. Romanian Biotechnology Letters. 14: 41644169. 10. Saier Jr MH, Trevors JT. (2010). Phytoremediation, Water, Air and Soil Pollution, 205 (Suppl 1), pp S61-S63. 11. Revathi K, Haribabu TE, Sudha PN. (2011). “Phytoremediation of chromium contaminated soil using Sorghum plant”. Int. J. Environ. Sc., 2(2): 417-428. 9 12. Pulford ID, Watson C. (2003). “Phytoremediation of heavy metal-contaminated land by trees-a review”. Environ. Internat. 29: 529-540. 13. Susarla S, Medina VF, McCutcheon SC. (2002). “Phytoremediation: an ecological solution to organic chemical contamination”. Ecological Eng. 18: 647-658. 14. Jadia CD, Fulekar H. (2008). “Phytotoxicity and remediation of heavy metals by fibrous root grass (sorghum)”. J. Appl. Biosciences. 10: 491 – 499. 15. Zhang S, Chen M, Li T, Xu X, Deng L. (2010). “A newly found cadmium accumulator Malva sinensis Cavan”. Journal of Hazardous Materials. 173: 705-709. 16. Salt, D. E., R. D. Smith, and I. Raskin. 1998. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 643-668. 17. Prasad MNV, Greger M, Landberg T. 2001. “Acacia nilotica L. bark removes toxic metals from solution: Corroboration from toxicity bioassay using Salix viminalis L. in hydroponic system”. International Journal of Phytoremediation. 3(3): 289-300. 18. Jin-Hong Q, Zayed QA, Yong Liang Zhu, Mei Yu, Terry N, Qian JH (1999). “Phytoaccumulation of trace elements by wetland plants: III. Uptake and accumulation of ten trace elements by twelve plant species”. J Environ Qual. 28:1448–55 19. Farago ME, Parsons PJ. (1994). “The effects of various platinum metal species on the water plant Eichhornia crassipes (MART.)”. Chem. Spec. Bioavail. 6: 1-12 20. Dushenkov V, Kumar PBAN, Motto H, and Raskin I. (1995). Rhizofiltration: the use of plants to remove heavy metals from aqueous streams. Environmental Science and Technology. 29: 1239-1245. 21. Matlwaska (2002). “Flavonoid compounds in the flowers of Abutilon indicum (Linn.) Sweet”. Acia Poloniac Pharmaceutic - Drug Research. 59 (3): 227–229 22. Rajakaruna N, Harris CS, and Towers GHN. (2002). “Antimicrobial activity of plants collected from serpentine outcrops in Sri Lanka”. Pharmaceutical Biology. 40: 235-244 23. Gamble JS. (2008). “Flora of the presidency of Madras”. Bishen Singh Mahendra Pal Singh Publishers. 23-A, New Cannaught Place, Dehra Dun – 248001 (India). I. 91. 24. Yoon J, Cao X, Zhou Q, Ma LQ. (2006). “Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site”. Sci. Total Environ. 368: 456-464 25. Ghosh M, Singh SP. (2005). “A review on phytoremediation of heavy metals and utilization of its byproducts”. Applied Ecology and Environmental Research. 3(1): 1-18 26. Cui S, Zhou Q, Chao L. (2007). “Potential hyper-accumulation of Pb, Zn, Cu and Cd in endurant plants distributed in an old smeltery, northeast China”. Environmental Geology. 51: 1043-1048 27. Li MS, Luo YP, Su ZY. (2007). “Heavy metal concentrations in soils and plant accumulation in a restored manganese mineland in Guangxi, South China”. Environmental Pollution. 147: 168-175 10