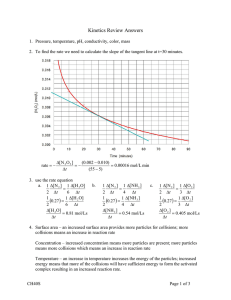
Collision Theory 1. Consider the following four reactions: (i) Fe(s) + I2(s) FeI2(s) (ii) C6H12O6(s) +6O2(g) 6CO2(g) + 6H2O(g) (iii) Ba2+ (aq) + SO42- (aq) BaSO4(s) (iv) H2(g) + Cl2(g) 2 HCl(g) a) Which of the four would likely have the fastest rate at room temperature? Explain your choice in terms of collision theory. H2(g) + Cl2(g) 2 HCl(g) – assuming the concentrations are the same the gas particles would be moving faster and would therefore collide more often b) Which of the four would likely have the slowest rate at room temperature? Explain your choice in terms of collision theory. Fe(s) + I2(s) FeI2(s) – very few collisions would occur so reaction would be the slowest 2. Given the following reaction 2 Al(s) + 3 F2(g) 2 AlF3(s) State five ways of increasing the rate of the reaction and use collision theory to briefly explain why it would increase the rate. (Assume the reaction is occurring in a closed container whose volume can be changed) Increase the temperature – will increase the number of collisions as well as the percentage of effective collisions as more of the particles will have enough energy to overcome the activation energy Increase the surface area of the aluminum – will increase the number of collisions Rates of Reaction For the reaction A + 3B C + 2D 1. Draw a graph that shows how the concentration of A will change as reaction proceeds? 2. Using the same graph show how the concentration of D will change as the reaction proceeds? 3. Using your graphs from above, state whether a reactions rate will be greatest at the beginning, at the midway point, or near the end of the reaction. Explain your selection. 4. Briefly explain how to calculate the average rate of reaction and the instantaneous rate of reaction using your graphs. Rate Law The experimental data below are obtained for the reaction a) b) c) d) e) Trial Initial [A] (mol/L) Initial [B] (mol/L) Initial [C] (mol/L) 1 2 3 4 0.10 0.20 0.10 0.20 0.10 0.10 0.30 0.10 0.10 0.10 0.10 0.20 2A + B + 2C 3X Rate of Production of X (mol/Ls) 3.0 x 10-4 1.2 x 10-3 3.0 x 10-4 2.4 x 10-3 What is the order of reaction with respect to each of the reactants? Write an expression for the rate equation. Calculate a value for the rate constant, k. Rewrite the rate law to include your k value. Calculate the rate of production of X when [A]=[B]=[C]=0.40 mol/L Potential Energy Diagrams For a reaction, the potential energies are as follows: activated complex, +122 kJ; reactants, +43 kJ; products, +74 kJ. a) Determine the activation energy (fwd and rev) and the enthalpy change for the reaction. Show all calculations. b) Draw a labeled potential energy diagram for the reaction, indicating the relative energies of the reactants, products, and activated complex, Ea (fwd and rev), and enthalpy. c) Is this reaction endothermic or exothermic? d) What would happen to your graph if a catalyst was added? Explain. Add this to your graph in another colour. Elementary Steps and Reaction Mechanisms 1. Identify the role of each of the following in the reaction mechanism below a) P2 b) PQ c) P2Q2 d) Q2 Step 1: P + R2 PR + R Step 2: R + Q2 RQ + Q Step 3: Q + PR RQ + P e) What is the overall reaction equation? (1 mark)