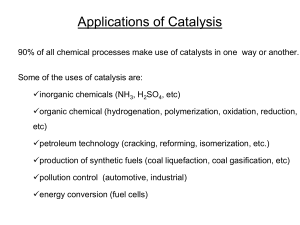
010715201603Abstract
advertisement

Room temperature C-H activation without Using External Oxidant: A Dual Catalytic Approach Manoj Kumar Sahoo† and Ekambaram Balaraman†* † Catalysis & Inorganic Chemistry Division, CSIR-National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune 411008, INDIA e-mail: mk.sahoo@ncl.res.in Introduction: Design of new catalytic reactions for selective organic transformations is essential for the sustainable production of chemicals. In recent years, merging of visible-light photoredox catalysis with transition-metal catalysis have drawn much attention and is an emerging area that can enable for unique reactivity and selectivity not observed using either catalyst independently. Here we report a ruthenium photoredox catalyst in tandem with a palladium catalyst effects the C-H activation of anilides with an array of aryldiazonium salts under very mild conditions; visible light, ambient temperature, no stoichiometric oxidant (Scheme 1). The broad substrate scope, mild reaction condition, readily available feedstock starting materials, and excellent regioselectivity of this process make it attractive for the effective synthesis of a wide range of N-heterocyclic moieties under environmentally benign conditions. Scheme 1. C-H activation using aryldiazonium salts by merging of visible-light photoredox catalysis with Pd catalysis 1