Enzymes - Dignam
advertisement

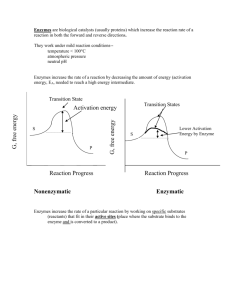
Dignam – Enzymes Reaction rate is accelerated; equilibrium distribution (and thus, equilibrium constant) of reactants and products is not changed types o oxidoreductases o tranferases (e.g. synthases) o hydrolases o lyases o isomerases o ligases (e.g. synthetases) Transition state o if increase concentration of TS, will increase rate of reaction Equilibrium is reached much more rapidly in the presence of an enzyme, but the equilibrium constant is not changed entropy – the ability of a system to occupy many different states o may use energy to constrain this number of states a decrease in entropy o a reaction that results in a decrease in enthalpy and an increase in entropy is a favorable one ABC (acid-base catalysis) o in a non-polar environment, acidic and basic residues exhibit increased activity via altered pKa Nucleophilic (covalent) catalysis o electron pair of nucleophile attacks electron deficient substrate o e.g. DNA topisomerase I Active site is often hydrophobic (lower dielectric constant stronger electrostatic interactions) Ribonuclease o residues in the active site general acid/base: H119, H12 stabilization of negative charge on the phosphate: K41 Aspartyl proteases (use of ABC) o e.g. pepsin, HIV protease, renin o involve pairs of carboxyl groups on aspartyl side chains to cleave peptide bonds the first residue acts as a base and abstracts a proton, while the second acts as an acid and protonates the nitrogen Serine proteases o e.g…. o chymotrypsin bulky and hydrophobic residues acyl intermediate catalytically active residues : S195, H57, D102 S195,H57 = acid/base catalysis D102 = uses H bond to orient H57 with S195 o S195 forms ester with substrate (nucleophilic catalysis) catalysis is a series of steps bind substrate, form tet. intermediate, release, add water (hydrolyses E-S bond) and reform catalytic portions, form second tet. intermediate, release, reform original catalytic portions o trypsin positively charged residues o elastase small and aliphatic residues Michaelis-Menten Equation o when [S] is small, reaction is almost 1st order o when [S] is large, v = vmax Dignam – Enzymes cont’d Briggs-Haldane Steady-State Approximation o more general o Km = (kcat + k-1)/k1 Double Reciprocal Equation o Y = 1/v o m = Km/Vmax o X = 1/[S] o b = 1/[Vmax] Enzyme inhibitors o Competitive same site bound by inhibitor and substrate effect may be reversed by increasing [substrate] apparent Km for substrate is increased velocity of reaction doesn’t change with increasing [inhibitor] double reciprocal plot shows same y-intercept, but greater slope o Noncompetitive velocity decreases with increasing [inhibitor] double reciprocal plot shows same x-intercept, but greater slope o Multi-reactant systems sequential ping-pong o “Km” and “Turnover number” slides to be on blackboard o Irreversible inhibitors covalent bond with some part of enzyme inactivation of enzyme interferes with an AA involved in S binding or catalysis o Suicide inhibitors substrates that are converted (by the enzyme) to irreversible inhibitors e.g. allopurinol xanthine oxidase (for gout treatment) Enzyme regulation [S], allosteric effectors (+ or -), covalent mods (+ or -), [E] (mRNA) Allosterism and cooperativity o allosteric effectors may affect Vmax and Km homotropic, heterotropic Enzymes and disease o mutations inactivity, instability, defective mRNA, promoter inactivation o deficient activity ACE inhibitors CPK isozymes o CPKMB levels rise following an MI, but not after other events (post-cath., abd. surg., etc.) Dignam – Myoglobin, Hemoglobin, and Enzymes Myoglobin o Heme – held in hydrophobic pocket Fe-protoporphyrin IX heme binds iron when it is in its ferrous (Fe2+) oxidation state oxygen binding site o F8-His, E7-His o O2 saturation dissociation constant = Kd = concentration at which 50% saturation is achieved Hemoglobin o effector sites: protons, 2,3-BPG, CO2, O2 o O2 saturation plot – know how the curve shifts Hill Equation – ligand binding to multiple sites o indicates extent of cooperative binding, and if the binding is positive or negative o know what the curve should look like if no cooperativity (n = 1), positive cooperativity (n > 1), or negative cooperativity (n < 1) o larger K of Hb allows the protein to respond to the need for oxygen (as opposed to the smaller K for Mb) Monod model for positive cooperativity of O2 binding to Hb o R state – relaxed; high affinity o T state – taut; low affinity