Effect of solution chemistry on flotation of pyrite in saline waters
advertisement
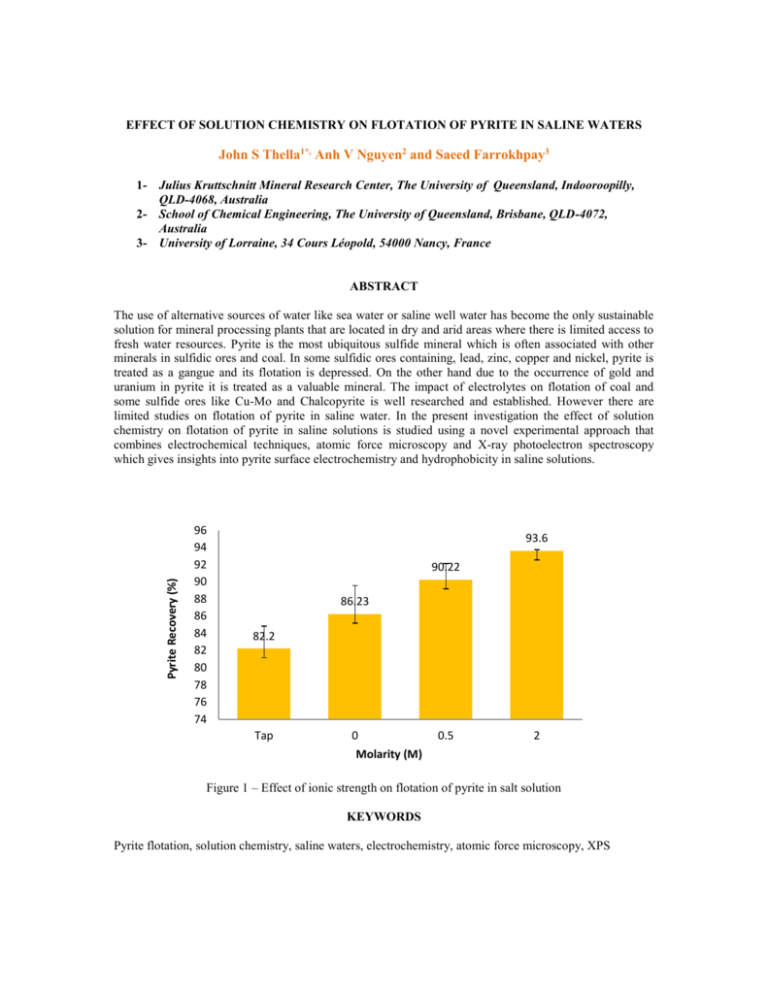
EFFECT OF SOLUTION CHEMISTRY ON FLOTATION OF PYRITE IN SALINE WATERS John S Thella1*, Anh V Nguyen2 and Saeed Farrokhpay3 1- Julius Kruttschnitt Mineral Research Center, The University of Queensland, Indooroopilly, QLD-4068, Australia 2- School of Chemical Engineering, The University of Queensland, Brisbane, QLD-4072, Australia 3- University of Lorraine, 34 Cours Léopold, 54000 Nancy, France ABSTRACT Pyrite Recovery (%) The use of alternative sources of water like sea water or saline well water has become the only sustainable solution for mineral processing plants that are located in dry and arid areas where there is limited access to fresh water resources. Pyrite is the most ubiquitous sulfide mineral which is often associated with other minerals in sulfidic ores and coal. In some sulfidic ores containing, lead, zinc, copper and nickel, pyrite is treated as a gangue and its flotation is depressed. On the other hand due to the occurrence of gold and uranium in pyrite it is treated as a valuable mineral. The impact of electrolytes on flotation of coal and some sulfide ores like Cu-Mo and Chalcopyrite is well researched and established. However there are limited studies on flotation of pyrite in saline water. In the present investigation the effect of solution chemistry on flotation of pyrite in saline solutions is studied using a novel experimental approach that combines electrochemical techniques, atomic force microscopy and X-ray photoelectron spectroscopy which gives insights into pyrite surface electrochemistry and hydrophobicity in saline solutions. 96 94 92 90 88 86 84 82 80 78 76 74 93.6 90.22 86.23 82.2 Tap 0 0.5 2 Molarity (M) Figure 1 – Effect of ionic strength on flotation of pyrite in salt solution KEYWORDS Pyrite flotation, solution chemistry, saline waters, electrochemistry, atomic force microscopy, XPS