formation of a scorodite-like mineral coating on pyrite and its
advertisement

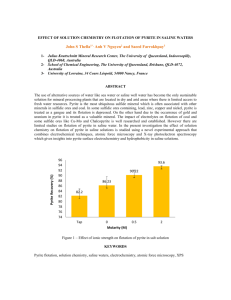
FORMATION OF SCHWERTMANNITE- AND SCORODITE-LIKE MINERAL COATINGS ON PYRITE AND ITS IMPLICATIONS IN ACID MINE DRAINAGE CONTROL ABSTRACT Acid mine drainage (AMD), the very acidic and heavy metals-contaminated leachate produced when pyritebearing rocks are exposed to oxidizing conditions, is a serious environmental problem encountered after the closure of mines and mineral processing operations. Although numerous researches have been published related to pyrite oxidation and AMD treatment, an economically sustainable solution to this problem is still out of reach. Moreover, the majority of these studies were done with freshly cleaved or polished pyrite rather than the weathered ones typically found in the environment. Long-term exposure of pyrite to air and moisture enhances the formation of secondary mineral phases like soluble salts and metallic oxyhydroxides that could play significant roles in pyrite oxidation dynamics and AMD generation. In this study, we investigated the effects on pyrite oxidation of soluble phases and metallic oxides commonly found in strongly weathered pyritic wastes. The pyrite sample used in the study came from Furikusa mine, Japan, contains minor amounts of arsenopyrite, and had been exposed to air and moisture for a very long time. This long-term exposure resulted in the formation of considerably thick salt-like mineral coatings composed mainly of iron, sulfur and oxygen with trace amounts of calcium, potassium, silicon and arsenic (Fig. 1(a)). Our results showed that soluble salts could either enhance or retard the oxidation of pyrite because of their strong influence on the leachate pH as well as in the formation of secondary mineral phases like scorodite, jarosite or schwertmannite. When the amounts of soluble salts decreased, a mineral-like coating formed on the pyrite surface that suppressed pyrite oxidation. Moreover, formation of this coating was apparently promoted after the addition of hematite into the system (Fig. 1(b)). This coating material, composed of iron oxyhydroxysulfates and arsenates, was mechanically strong enough to withstand the constant shaking motion of the experiments, and could be potentially used as precursor materials to passivate pyrite with iron oxyhydroxides that are very stable under surface oxidizing conditions. Figure 1 – (a) a 3D photomicrograph of the salt-like coating formed on pyrite, and (b) the schwertmanniteand scorodite-like mineral coating formed after 21 days KEYWORDS Acid mine drainage, Pyrite, Arsenopyrite, Pyrite oxidation, Pyrite passivation