Diapositiva 1 - CETEM - Centro de Tecnologia Mineral
advertisement

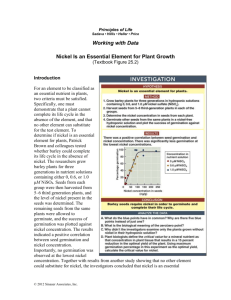
Experimental Design to evaluate a reagent system for a nickel ore flotation Authors • Jean Louzada; Ronald Hacha; Marisa Monte and Mônica Cassola • (a) CETEM – Centre for Mineral Technology, Rio de Janeiro, Brazil • (b) Clariant S.A, São Paulo, Brazil MOTIVATION •The optimization of flotation conditions is a complex task because many process variables can affect flotation responses. •It is not uncommon for multiple interactions to occur between independent variables; •The identification of these interactions play an important role in advancing our understanding of the chemistry of such system in plant operations. OBJECTIVES • To employ a factorial design to investigate the effect of chemical variables on the flotation performance of dithiophosphates for a nickel ore; • To optimize these variables for maximum nickel recovery and grade. EXPERIMENTAL – REAGENTS – Ethyl secbutil sodium dithiophosphates (Hostaflot E501) and sodium dialkyl dithiophosphate (Hostaflot M92) were supplied by Clariant; – Polypropylene glycol methyl ether (CH3(OC3H6)n-OH and other consisting of a mixture of aliphatic alcohols, ethers and esters. The two frothers were supplied by Clariant . – The activator and the depressant used were copper sulfate and carboxymethyl cellulose, respectively EXPERIMENTAL – A nickel ore sample from Minas Gerais, Brasil, was completely characterized for mineralogical and chemical compositions: • Mineralogical composition, associations and liberation were measured in a FEI Quanta 400 SEM with the Mineral Liberation Analyzer (MLA) software. • Chemical analysis were carried out in a PanAnalytical Epsilon 3 X-ray Fluorescence machine. EXPERIMENTAL – Factorial Design; • Only factors which influenced in the recovery of nickel by flotation will be presented here. • The factorial design was implemented with two levels and six factors resulting in thirty two experiments. • The Statistics software was used for the regression analysis, statistical and optimization calculations. Table 1. Factors and levels applied in 26-1 fractional factorial design. Flotanol (low: 20; high: 40); Montanol (low: 20; high: 80). Parameters Values Factors Symbols Units Low level (-1) High level (+1) Collector Type A -- Hostaflot M92 Hostaflot E501 Collector Concentration B (g/t) 80 200 Frother Type C -- Flotanol Montanol D (g/t) Low High E (g/t) 50 100 F (g/t) 50 100 Frother Concentration Dispersant Concentration Depressant Concentration Experimental • Flotation Tests • • • • • • • The samples were ground in a rod mill, to which were added the dispersant and the activator at pH 6.0. Immediately after grinding, the material was deslimed and, subsequently, the sample was transferred to a cell with two liters. The pulp was kept under stirring at 1400 rpm and the pH was adjusted to 9.5 with a solution of NaOH 10% (p/v). pH adjustment was immediately followed by depressant addition, carboxymethyl cellulose, and conditioning for 4 minutes. Afterwards, the collector was added and conditioned for 30 seconds. Finally, the frother was added to the system and conditioned for 1 minute. The pH was kept at about 9.5 during the conditioning with all reagents. The flotation time was 4 min. Experimental – Curve Fitting and Statistical Analysis • The important response variable chosen in this study was nickel recovery • The statistical significance of effects and interactions between processes and the response variable was determined using the F-test. • Probability (P) values larger than 0.05 were indicative of a measured effect being statistically significant at a confidence level 95% RESULTS AND DISCUSSION Mineralogical characterization • These studies showed that the major minerals are talc, hornblende, ilmenite, pyrite and pyrrhotite. • The results revealed that talc is not the predominant magnesium carrier mineral • Hornblende is present and predominates over talc in all ranges of particle size. Figure 1 Mineralogical Composition as a function of the particle size Talc Apatite Chlo rite1 Ilmenite Pyro xmangite Titanite Pentlandite Calc ite Do lo mite K_feldspar_1 Pyro xmangite_Pyro xf Magnesio ho rnblende Carbo nato FeMg FeO Plagio c lase1 Pyrrho tite Ankerite Chalc o pyrite Ferro silite Pyrite Quartz 100% 90% 80% 70% Massa (%) 60% 50% 40% 30% 20% 10% 0% -300+210 -210+150 -150+106 -106+75 Fração (um) -75+53 -53+38 Figure 2 Minerals associated to pentlandite in the particle size range between 210 and 38 μm. Talc 12% Magnesiohornblende 3% Ankerite 2% Free Surface 52% Calcite 4% CarbonatoFeMg 2% Chlorite1 3% Dolomite 0% Ferrosilite 5% Pyrite 3% Quartz 1% Pyrrhotite 6% Pyroxmangite_Pyroxf 5% Figure 3 - Synthesis of the results obtained for theoretical recovery and grades of pentlandite in the concentrate at different size ranges. Pareto Chart Pareto Chart of Standardized Effects; Variable: Recuperation of Ni (%) 6 factors at two levels; MS Residual=12.37447 DV: Recuperation of Ni (%) B D B*D A*C C*E C C*D A*C*D E A*B*C B*C C*F B*C*E A*F A A*B*E A: Type of colletor B: Concentration of C:Type of frother D: Concentration of E: Concentration of F: Concentration of A*B*F B*C*D B*E B*C*F A*B*D p=.05 Standardized Effect Estimate (Absolute Value) colletor frother disperst depressor Analysis of Variance These variables and their interactions presented higher probabilities: • B: Concentration of collector ; • D: Concentration of frother • BD: interactions between them • In other cases, the null hypothesis is rejected because the estimated values of p-levels (Test P) are smaller than 0.05, i.e., the effects have a probability smaller than 5% so they represent only of noise. Response Surface . Recu peration of Ni (%) (%) Nickel Recovery Fitted Surface; Variable: Reuperation Nickel Recovery of Ni (%)(%) 6 factors at two levels; MS Residual=14.1346 DV: Recuperation Nickel Recovery of (%) Ni (%) Co nc Fern t ortha et iro n C oonf cfer ont r hae trio (gn ( /t)g/ t) tt)) (g (g/ / r n o ctti o lltera o n c f ce nCoo n o i r t tracto eonll e c nC Co CONCLUSIONS • The results of these studies showed that the main factors that influence more significantly the nickel recovery are the collectors and frothers concentrations. • The differences between the collectors are the alkyl chains do not influence the recovery.